Ethylene, the main intermediate feedstock for petrochemical production, with diverse uses and significant consumption in Ghana, is imported for use despite the availability of raw materials for its production. This paper aims to assess the technical feasibility and economic viability of producing olefins like ethylene and propylene from recovered ethane gas at the Gas Processing Plant (GPP) in Ghana; to optimise Ghana’s hydrocarbon resources. The steam cracking method of olefin production was chosen for this research after a review of pertinent literature and a general assessment conducted. The basis of this selection was its widespread use; thus, easily accessible facilities, comparably anticipated lower costs to be incurred, more stable products formed, and minimal environmental effects after comparison with the Oxidative Dehydrogenation of Ethane (ODHE) and Catalytic Ethane Dehydrogenation (EDH) methods. With the aid of Aspen Hysys Simulation Software V11, this paper was carried out by utilizing process simulation techniques to design an olefin production plant. Using the Aspen Process Economic Analyzer and Microsoft Excel Spreadsheet Application, cost estimation and economic analysis were conducted while applying standard investment decision criteria. Based on a successful simulation and results obtained with a Net Present Value of $19,343,264.87, an Internal Rate of Return of 27% and a Payback Period realized at year 3, the paper concluded that the venture portrayed technical and economic attractiveness.
Keywords: Ethylene, Olefins, Economic viability, Processing plant, Steam cracking
Once only a great nuisance, commercial petroleum has become the critical lifeblood of the industrial world; with wars being waged for this carbon prize. The great hydrocarbon rush now causes producing countries' governments to reign by its value while consuming countries' economies are being subjected to its price.1 With the emergence and increasing importance of natural gas, economies have invested in its production and consumption, not only because of its environmental friendliness but also because of the current and anticipated profit upswing; and Ghana's economy is no exception.2 Ghana has sig- significantly optimised her gas resources by producing, processing and advocating for citizen use of natural gas resources for thermal power generation, combined heat and power by industrial companies, and liquefied petroleum gas as a replacement for cooking and other domestic purposes.2
The goal of this paper is to investigate the technical feasibility and economic viability of locally producing olefins such as ethylene and propylene, the main intermediate feedstock used to produce various petrochemical products; from ethane gas recovered at the Gas Processing Plant in Ghana.
The focus of such an investment is to optimise ethane gas, which currently has no distinct market in Ghana (and as a result, is comingled with methane gas to serve as lean gas), to produce olefins (ethylene and propylene) which have a considerable market and are presently imported for use by numerous companies.3
This paper seeks to explore and examine the industrial method(s) of large-scale olefin production from ethane gas; in order to select the most feasible and applicable method; to simulate an olefin production plant using recovered ethane gas conditions; and subsequently to conduct an economic assessment of the possible investment to determine profitability.
Statement of theory
Following its commercial discovery, the Ghanaian government, through the Environment Protection Agency, prohibited gas flaring except for emergency or short-term operating needs. In this vein, Ghana has used her natural gas resources from both regional and domestic sources for power generation, industrial power, and heating, as well as for residential activities such as cooking (Liquefied Petroleum Gas).
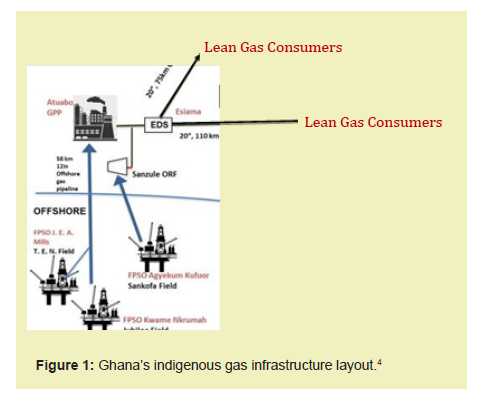
The Gas Supply Sources in Ghana are listed as follows and shown in Figures 1 and 2:
- Domestic (Indigenous) Source - Associated gas processed and recovered at the Gas Processing Plant upon receipt from the Jubilee and TEN Fields and non-associated gas from the Sankofa- Gye Nyame Field.
- Regional Source - West African Gas Pipeline Company.
As displayed in Figure 1, indigenous lean gas obtained from the Gas Processing Plant is transported to consumers through transmission pipelines for power generation and industrial purposes. Other gas products (LPG and Condensate) recovered at the plant, make their way to the final consumer via trucks from a loading gantry.
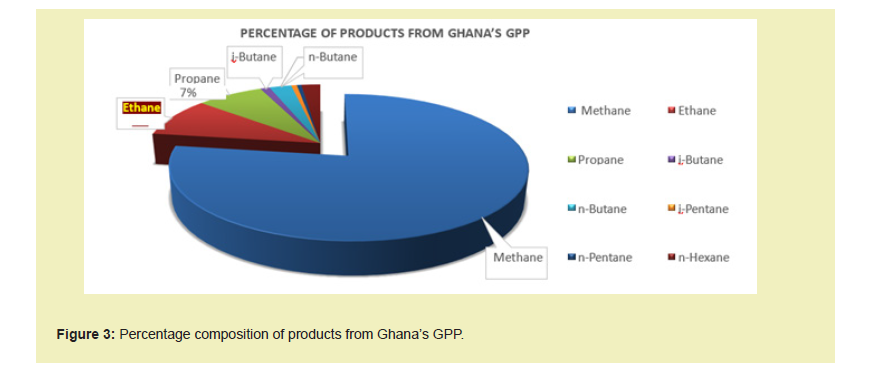
Ethane gas, as illustrated in Figure 3, represents 9% of products recovered at the Processing Plant. This percentage, equivalent to a daily volume of 10.811 MMscf currently, is comingled with the methane fractions and commercialized as lean gas to serve the purpose of power generation and industrial heating.

Not only will olefin (ethylene and propylene) production from the recovered ethane gas readily provide ethylene for the fifty-two (52) identified importers of ethylene in Ghana,4 but also ensure the durability of turbines of power producers, who represent about 84% of lean gas consumers in Ghana; and whose facilities have been designed to utilize methane gas of specified heating value (1110 – 1135 Btu/SCF) for power generation.
This step will also be an attempt to minimize imports of the various polymers of ethylene for the myriad of purposes they serve in packaging, health, oil and gas processing, agriculture, and various other sectors of the Ghanaian economy Figure 4.
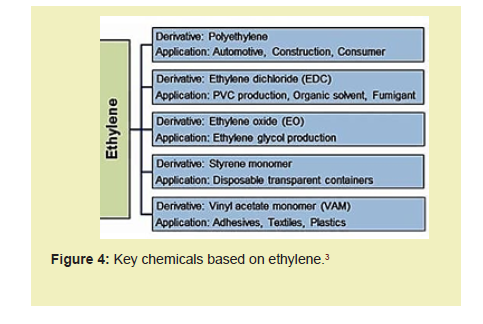
The discussion as follows compares the Steam Cracking Method, Catalytic Ethane Dehydrogenation, and Oxidative Dehydrogenation of Ethane (ODHE) methods:
Steam cracking
Steam cracking is a petrochemical process in which saturated hydrocarbons are broken down into smaller, often unsaturated, hydrocarbons. It is the principal industrial method for producing lighter alkenes (olefins), including ethylene and propylene. In steam cracking, a gaseous or liquid hydrocarbon feed like ethane or naphtha, is diluted with steam and then briefly heated in a furnace up to a temperature of about 850°C in the absence of oxygen. After this cracking temperature has been reached, resulting in the formation of unsaturated hydrocarbons (ethylene, propylene, butadiene, etc), the gas is quickly quenched to stop the reaction in a transfer line exchanger. Steam cracking produces a wide product distribution, however, the products formed in the reaction depend on the composition of the feed, the hydrocarbon-to-steam ratio, cracking temperature, and furnace residence time. Light hydrocarbon feeds (such as ethane, LPG, or light naphtha) give product streams rich in lighter alkenes, including ethylene, propylene, and butadiene.5 Cryogenic distillation is employed to obtain pure ethylene from the cracked gas after undergoing a series of compression, drying, and sweetening stages.6
Catalytic ethane dehydrogenation
In steam cracking/pyrolysis, the formation of side products such as aromatics and light gaseous hydrocarbons is unavoidable, although sustainable at high temperatures. The side processes involved in the formation of by-products reduce the efficiency of ethylene production. Catalytic ethane dehydrogenation over oxide or supported-metal catalysts allows for ethylene production at lower temperatures than pyrolysis of ethane. The reaction, however, is endothermic and thus, a high temperature and low pressure of ethane must be used in this process for high ethane conversion.
Platinum, Pt and Palladium, Pd-containing systems are common catalysts for selective hydrocarbon dehydrogenation. At low temperatures, ethane conversion over noble metal catalysts occurs. However, unsaturated Pt may induce side processes such as ethane hydrogenolysis and subsequent polymerization to coke which are undesired.6
Oxidative Dehydrogenation of Ethane (ODHE)
Oxidative dehydrogenation of ethane (ODHE) has been discovered to be an appealing alternative for producing ethylene that does not require internal heat input. It has been reported that molten alkali chlorides such as LiCl, KCl, NaCl, Li-K- Cl, Li-Na-Cl, Li-Sr-Cl, and Li-Ba-Cl supported by Dy2O3/MgO can produce ethylene with a yield of nearly 75%. However, this process can result in a variety of Cl-containing by-products that are considered environmentally hazardous and incur additional costs of treatment. Deactivation of catalysts (a temporal or permanent loss of active sites) also remains one of the major constraints of the ODHE method.6
Catalytic Ethane Dehydrogenation and Oxidative Dehydrogenation of Ethane, although they exhibit high selectivity to ethylene due to catalysts used, face significant catalyst deactivation due to the unstable nature of catalysts; resulting in excessive downtime for catalyst activation/replacement; and excessive environmental hazards due to the production of Chlorine-containing by-products which in turn incur high additional costs of removal.7 The products formed during steam cracking are highly affected by the composition of the feed and in this case, ethane gas (a more specific feed), which limits the issue of low selectivity. For this investigation, steam cracking is selected due to its wide usage and thus, readily available facilities, comparative environmental safety and anticipated lower cost to be incurred.
In the proposal this paper presents, the concept of ethylene production from ethane gas employed by Carbide and Carbon Chemicals Corporation, the first firm to produce ethylene from light hydrocarbons (predominantly ethane) is applied. The company, now a subsidiary of Dow Chemicals, owned a small plant in Clendenin, West Virginia, that separated natural gasoline from raw natural gas. The gasoline, which at room temperature is a liquid, was sold as fuel.
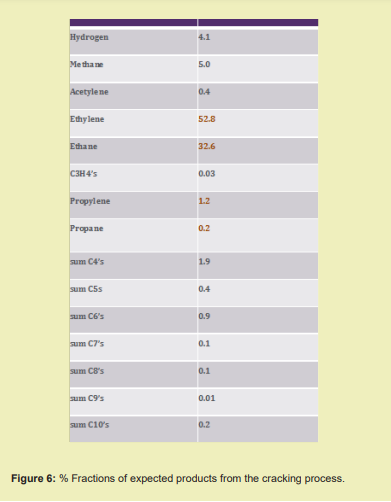
However, the remaining liquids derived from natural gas, consisting mostly of ethane and propane, found no ready markets, so the company installed processing units and a furnace to convert them into more valuable products. The furnace began operating in 1921 to produce ethylene8 Figures 5,6.


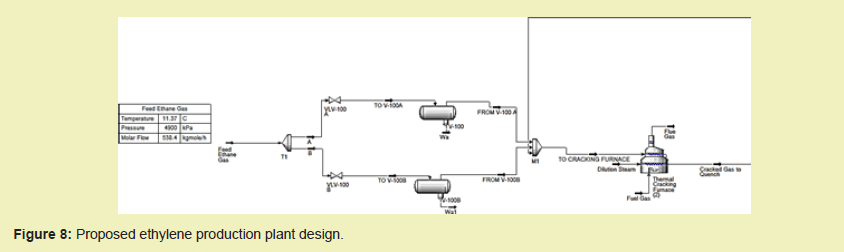
Aspen HYSYS, developed by AspenTech was used to create the design of the proposed ethylene production plant. After completion of the simulation, cost estimation was conducted using the Aspen Process Economic Analyzer (APEA) and data from the US Department of Energy. These costs were recorded using the Microsoft Excel Spreadsheet Application and further analysed.
The Process Simulation employed the Peng Robinson and SRK Fluid Packages; as feed streams and expected products were hydrocarbon in nature. Upon selecting individual equipment making up the entire proposed plant and defining their compositions as well as all material and energy streams, the simulation was run successfully. For some components, an N+1 redundancy was employed to ensure system availability in the event of component failure.7
The proposed design to be displayed in the next section consists of:
- Inlet Filter Separators to extract any entrained solid particles and moisture components from the feed gas
- The cracking section which primarily undertakes preheating of the feed ethane to about 120 °C by steam, subsequent heating to the incipient cracking temperature of averagely 600 °C in the convection section, and further to cracking temperatures of about 850 °C in the radiation section by fired tubular reactors. Afterwards, the cracked gas is cooled briefly by a Transfer Line Exchanger. A schematic of the cracking process is shown in Figure 7.
- The quenching section which employs the quench tower for drastic cooling of the cracked gas to about 180°C
- A series of five turbine-driven centrifugal compressors to compress the quenched gas ahead of fractionation
- An acid gas removal unit consisting of a caustic scrubber to extract acid gases in order to prevent the formation of ice and hydrates
- A dryer after the last stage of compression removes moisture and prevent ice and hydrates formation.
- A series of fractionation towers (depropanizer, demethanizer, deethanizer, C2 and C3 splitters) for the recovery of ethylene, propylene, and by-products
- Refrigeration of products.5,8,10

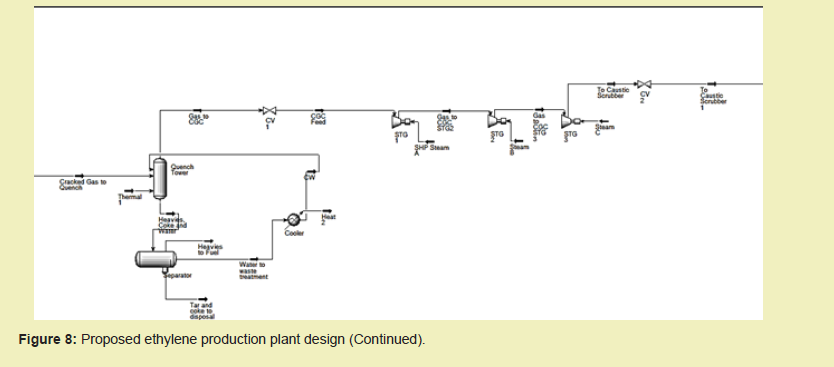
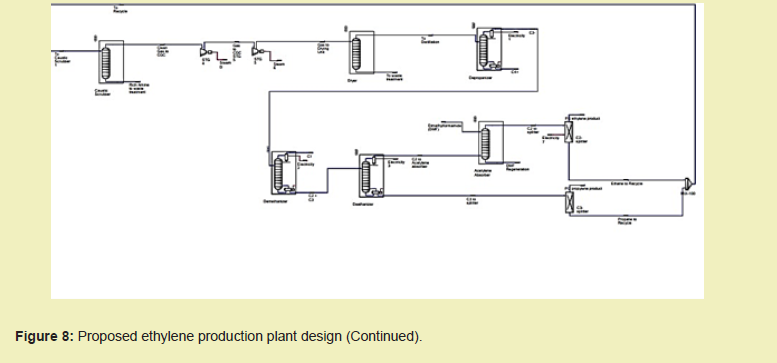
Find as follows, the proposed design of the ethylene production plant with components discussed in the previous section of this paper; Subsequently in this section, data pertaining to plant economics and assessments are provided.
Unreacted and product ethane and propane streams recovered from the C2 and C3 splitters are recycled by transporting them to the inlet of the Thermal Cracking Furnace.
- V-100 A/B -Filter Separators, CV and VLV-Valves, CGC-Cracked Gas Compression, STG- Stage
- T1, M1 and MIX 100 represent pipe spools
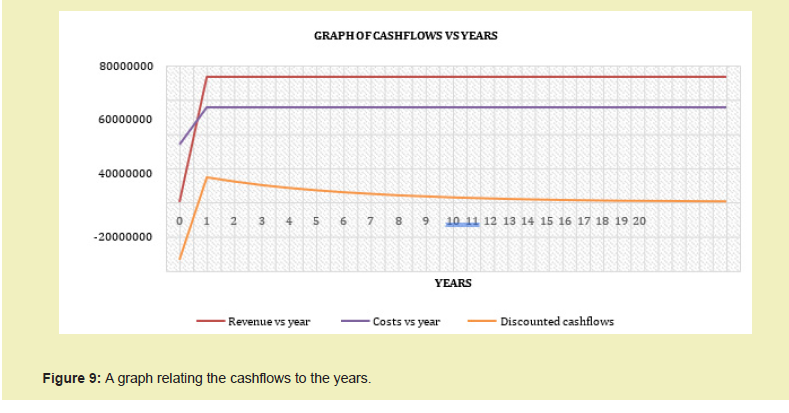
With individual costs of equipment and installation generated as presented in Appendix A and estimation of other costs conducted with cost components provided in Appendix B, find as follows, a summary of the plant economics Figure 8.
The Total Required Delivered Price estimated to be used as a benchmark for the pricing of the ethylene product obtained was found to be USD/mt 821.95.
Applying the current average prices of ethylene and propylene,6 estimated revenue is summarized as follows Tables 1,2:
TThe expected Total Daily Revenue to be obtained from the sales of ethylene and propylene was estimated at USD 210,948.26 and yearly revenue at USD 73,146,309.51. This was obtained by applying the current average prices of ethylene and propylene and the expected product recovery. A 5% allowance was given to cater for Turnaround periods.
After a literature review, general assessment, method selection, process simulation and subsequent analysis using investment decision criteria, it is concluded that;
- The steam cracking method is currently the most feasible method of ethylene production to be possibly executed in Ghana with the proposed plant design illustrated in Figure 7.
- By employing the NPV investment decision criteria, ethylene production in Ghana is determined to be a viable venture.
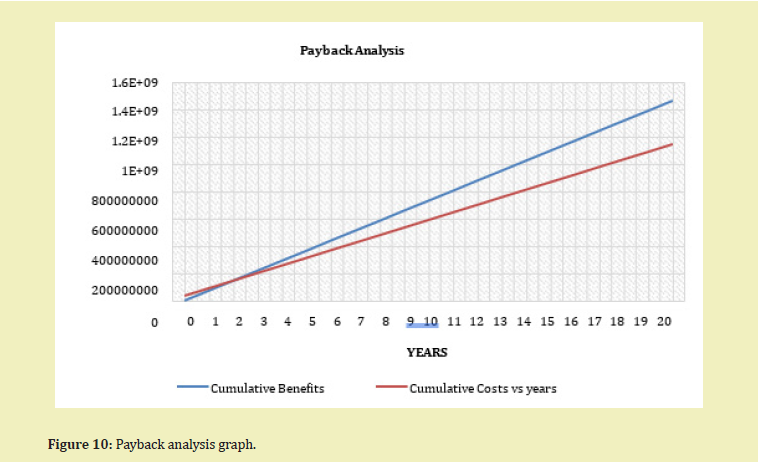
- With an investment worth USD 33,801,257.88 as Total Fixed Investment and USD/mt 821.95 Total Required Delivered Price, a Net Present Value of USD $19,343,264.87 an Internal Rate of Return of 27% would be realized at the end of 20 years with profit realization starting at year 3.
- Investment into this venture will aim at reducing the dependency of our local petrochemical production companies on imported ethylene, generating income for the country, providing employment, and providing leaner gas for the power-generating companies.
The authors wish to express their sincere appreciation to the faculties at the Department of Petroleum and Natural Gas Engineering, University of Mines and Technology, Tarkwa, Ghana for their immense support.
This Research Article received no external funding.
Regarding the publication of this article, the authors declare that they have no conflict of interest.
- 1. Waples DA. The Natural Gas Industry in Appalachia: A History from the First Discovery to the Tapping of the Marcellus Shale. McFarland, Jefferson. 2014;2.
- 2. Anon. Petrochemical Plant Economics. 2023.
- 3. Alshammari A, Kalevaru VN, Bagabas AA. Production of Ethylene and its Commercial Importance in the Global Market. 2016.
- 4. Anon. “Final-The 2021 Gas Challenge Study Guide-Approved, Tertiary Institutions Edition”. Ghana National Gas Company Limited.
- 5. Gärtner CA, Veen ACV, Lercher JA. Oxidative Dehydrogenation of Ethane: Common Principles and Mechanistic Aspects. Chemistry Euroope. 2013;5(11):3196-3217.
- 6. Fairuzov D, Gerzeliev I, Maximov A, et al. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Chemical Looping for Catalysis. 2021;11(7):833.
- 7. Anon. “Ethylene Gas Buyers in Ghana”.
- 8. Stone D. Birth of the Petrochemical Industry. American Chemical Society.
- 9. Fernández L. Price of Ethylene Worldwide from 2017 to 2022.
- 10. Posch W. Applied Plastics Engineering Handbook. Elsevier Inc. 2011:p.23-48.