Since the year 2000, we have been generating vinyl acetate-acrylic acid (VA-AA) copolymers from the free-radical retrograde-percipitation polymerization (FRRPP) process, and we have been formulating them as multipolymeric surfactants for various applications. This work involves the use of the Microtox® method to investigate the aquatic toxicity behavior of the aqueous emulsions of VA-AA-based multipolymeric surfactants, and their formulations within liquid-liquid and solid-liquid interfaces. Liquid-liquid and solid-liquid interfaces employed in this study are represented by crude oil-water and bauxite extraction waste (Red mud)-water mixtures, respectively. Investigations with these interfaces reveal that the VA-AA-based multipolymers either reduce or eliminate the nascent aquatic toxicities in these mixtures.
Keywords: Vinyl Acetate-Acrylic Acid copolymer, Polymer surfactants, FRRPP process, Microtox, Crude oil, Red mud, Aquatic toxicity
Surfactants find a wide range of uses based on their unique properties, particularly their influence on interfacial surfaces. Few examples are enhanced oil recovery in petroleum industry, engine oil separations in automotive industry,1 and detergents in textile industry and as a floating agent in ore extraction processes. Because of their distinctive macromolecular structure, they are able to adhere or adsorb on interfaces, which result in lesser application of energy for separation of the phases.
Specific properties of surfactants are due to their typical molecular structure which comprises of two different and operationally opposite structures, called as lyophobic or the hydrophobic group and lyophilic or hydrophilic group. The hydrophobic group is the tail part and hydrophilic is the head part of a typical surfactant structure. In immiscible aqueous solutions, such structures successfully remain attached to both phases according to the affinity of the group structure. For example, where water is the solvent, the hydrophobic part is responsible for distortion of the water phase causing increase in free energy of the system, hence requiring less work to be done on the system for phase separation. At the same time the hydrophilic part remains attached to the water phase preventing the surfactant to be excluded from the system.1
Hydrophobic groups in surfactant molecules are usually long chain hydrocarbons, whereas the hydrophilic part relies on strong specific interactions, such as polar, hydrogen-bonded, and ionic groups. There are four basic classifications of surfactants based on the nature of the hydrophilic head. The following classifies commercial surfactants based on the following functional groups:
- Anionic - The hydrophilic head part is a negatively charged group.
- Cationic - The hydrophilic head part is a positively charged group.
- Nonionic - The hydrophilic part does not have any charge.
- Zwitterionic - The hydrophilic part may have both positive and negative charges.2
Further classifications are also available based on nature of attached hydrophobic groups, and even the long-range macromolecular architecture of both hydrophilic and hydrophobic groups. Current research focusses on comparing various performance characteristics of vinyl acetate-acrylic acid (VA-AA) based copolymer surfactants with other commercially available surfactants. Typically, the so-called B6-1 VA-AA tapered block copolymer surfactant used in this work (Figure 1(a) and 1(b)) has the following CAS registry:3,4
- CAS # [903900-50-5]
- Type of polymer – a reduced regulatory requirement (RRR) polymer
- CAS Name of polymer: 2-Propenoic acid with ethenyl acetate, hydrolyzed
- Common Name: Partially hydrolyzed vinyl acetate-ammonium acrylate copolymer
- Molecular Structure:
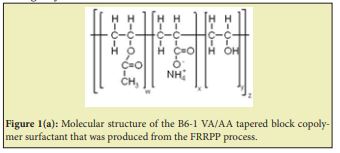
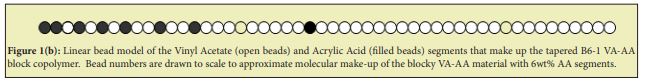
Other VA-AA-based in-house copolymer surfactants used in this work have similar structure to B6-1.
Toxic effects of effluents that concern people throughout the world include those being discharged into the environment from agricultural, construction, mining, chemical, health care and many other industries. Various biological assays have been utilized to determine toxicities of samples, such as those of mysid shrimp (Americamysis bahia) and inland silversides (Menidia beryllina),5 amphipod (Ampelisca abdita) and mysid (Americamysis bahia).6 Among these, Microtox® Acute Toxicity testing has become widespread because of its advantages in rapidity, simplicity, economic cost and portability.7-11 By comparison, conventional acute toxicity bioassays utilizing multi-cellular organisms are lengthy and involve complex and long exposure periods.12 The wide range of applications of Microtox® Acute Toxicity testing include assessments of surface or waste water, effluents, solid sediments, organic and pure compounds.
Surfactants with all their applications are also reviewed for their toxic effects on the mode of application they are performed. Here, we are aiming to work on using surfactants for oil spill herding and recovery, making dispersants for dispersion of crude oil, altering waste products into useful substances. Surfactants are basic constituents in these applications, which if applied to oil spills under requisite condition may contribute to acceptable damage to the affected environment.13 However, surfactants are found to be toxic to marine organisms in various studies conducted by researchers.13-18 A completely or relatively non-toxic surfactant will serve as boon for oil spill control purposes that are prevalent in water bodies. For completing this objective, it will be important to know the toxic effects of surfactants before and after application with different materials along with different solvents with crude oil compared to the effects of crude oil alone. Oil spill control formulations for oil spill dispersion contain significant amounts of surfactants in composition. The toxic contribution of surfactants in the formulation has to be studied. In-house synthesized surfactants along with some commercial surfactants, solvents to be used, dispersed crude oil were tested for Microtox® toxicity which utilizes special strain of marine bacterium Vibrio Fischeri (photobacterium phosphoreum),8,19 a luminescent bacteria, based on change in light intensities due to the sample that was exposed.10,20 The Microtox® Testing equipment was chosen because of its speed and portability, considering the possibility of testing of various upcoming potential dispersants and chemicals that might be used for oil spill dispersion or recovery in laboratory or the field. Also, it is as comparable screening test as long term exposure tests, to get an idea of the toxicity standings of dispersant chemicals21 as well as this method proves its suitability for toxic screening tests based on its comparative results with both vertebrate and invertebrate test results.13 The decision of implementing a surfactant or dispersant for sorted applications cannot be finalized based on this testing only. These screening tests play an important role however, and as an immediate response assessment.
Aluminum is a packaging material that is almost 100% recyclable at an infinite number of times, using only 5% of the energy it takes to produce it the first time. In its production from bauxite ore, Alumina is first extracted using a highly basic caustic solution using the Bayer Process,22 and the spent ore (tailings) is a red caustic particulate material that poses risks to human health, to the groundwater, livestock, and to the natural and built environment. Addressing the fate of waste materials from the extraction of Alumina from bauxite ore is needed to complete the sustainability characteristics of the use of Aluminum as packaging, structural and decorative material; note that Alumina is needed to produce Aluminum through the Hall Process.23 This is especially true in light of recent events in Hungary in the fall of 2010 when a red mud repository caused several deaths and massive ground and structural contamination.
The principal idea or purpose behind this work is to understand and compare toxic effects of various components of a surfactant formulation, for its prior and post applications, on crude oil using a handy acute toxicity measurement device. This Microtox® device is also used to compare aquatic toxicities of untreated and treated Alumina production tailings (called Red Mud) from bauxite ore, through the use of VA-AA-based copolymer surfactants. These tests reveal that the salt versions of these surfactants are relatively nontoxic in water, and in combination with highly caustic red mud materials. In mixtures with crude oil, the tests resulted in reduced aquatic toxicities compared to commercially available surfactants.
Materials and chemicals
The crude oil used in this work was light sweet crude from a Gulf of Mexico oilfield. Commercial surfactants were obtained from various company sources, and vinyl acetate-acrylic acid-based copolymer surfactants were produced in-house through the FRRPP process.3 Surfactant samples with 1% by weight aqueous solutions were prepared (up to 300ml), and stored in air tight containers. Red mud samples were obtained from blended cut samples of various operations in India, Jamaica, Venezuela, and Brazil (donated by Tailings Management Systems of Ontario, Canada). All reagents used were analytical grade, except for water that came from the distilled water source.
Apparatus and procedure
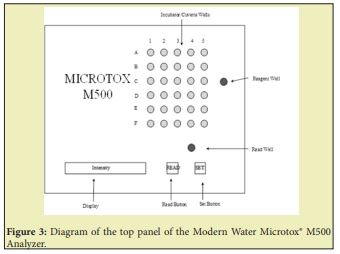
The Microtox® Test equipment (Figure 2) consists of Microtox® Model 500 (M500) (Modern Waters, Inc. of Newcastle, Delaware) analyzer which is a biosensor-based measurement system that uses bioluminescence technology to monitor harmful substances. It is a temperature-controlled, self-calibrating photometer that measures light intensities emitted by bioluminescent bacteria (Vibrio-Fischeri) in the presence of samples and reference standards. The decrease in light intensities as a result of the presence of toxic substances is related to toxicity values measured in terms of effective concentration at 50% of reduction in light intensity display at different times (typically, after 5 and 15 minutes of exposure) expressed as EC50 in “%” or “mg/l”. The device also consists of incubator system which is used to maintain temperatures of the “reagent well (1)” at 5oC and, “read well (1)” and “Cuvette or Incubator wells (30)” at 15oC, where reconstituted bacteria and testing samples are placed respectively. The tested data is calculated and stored using Microtox® Omni software which can be connected to the analyzer.

Three solutions obtained along with the system are Diluent, Reconstitution Solution and Osmotic Adjustment Solution (OAS). All the samples while testing was diluted at 1:2 by addition of diluents. Reconstitution solution is used for revival of the bacteria reagent after removal from the freezer. Diluent and Reconstitution solution are basically 2% NaCl solutions specially prepared with ultrapure non-toxic water, while OAS is a 22% NaCl solution used to adjust osmotic pressure of samples at higher concentrations. For sample, diluent and reagent transfers; the use of 3ml syringes, 10µl fixed pipettor and 500-5000µl variable pipettor and 0-1ml high precision syringes were used as required. 12x75mm2 cylindrical borosilicate glass cuvettes for testing are obtained from Modern Water Inc. From above materials the Microtox® model M500, Bacterial Vials, Microtox®Omni Software, Diluent, Reconstitution solution, OAS solution and cuvettes were obtained from Modern Water Inc. The Microtox® testing is based on following experimental design concerning important elements like sample state, Microorganisms used, dose type, end point determination and exposure period Figure 3.
Several samples were prepared and tested as per the Microtox® manual from AZUR Environmental, formerly Microbics Corporation (Modern Water Inc.).24 The samples were tested in duplicates at a time or twice to obtain EC50 values expressed as “%” values with 95% confidence range. The EC50 values have inverse relationship with toxic effect of the sample; implying higher EC50 values indicate low toxicity and vice-versa. The end points were at 15min or 10min (based on toxic effect) after addition of reagent to samples at different concentrations. The standard test was performed with Zinc Sulfate Heptahydrate as a standard before opening a reagent lot for testing.
Known concentrations of contaminants were prepared before conducting each respective test. The Microtox® M500 Analyzer is switched ON and some time is allowed for the Read well, Reagent well and Incubator wells to reach their required temperatures. The software Microtox® Omni is started and based on the sample, type of test is selected from a list shown on screen. Accordingly, cuvettes are placed in corresponding positions in the Incubator wells and the Reagent well. In the Microtox® system there are several tests that can be selected based on the sample concentration and expected toxicity values. The necessary tests for our samples are among 2% Basic Test, Basic Test (45%) and 81.9% Basic Test, where the percentages relate to the amount of concentration added in diluent for osmotic adjustments. We conducted the tests based on expected toxicity values and haziness factors.
A brief procedure for testing is shown as follows with appropriate and basic lab guidelines that are expected to be followed. The method is simple to perform since every instruction will be displayed by the software, but proper care while transferring and adding materials is the responsibility of the experimenter which is challenging to maintain. To start with a 2% Basic Test with 1 sample, 1 control and 4 dilutions or concentrations place cuvettes in Rows A and B along with a cuvette in the Reagent Well. Add 500µl diluent from B1 to B5 and 1000µl from A1 to A4. Add 100µl sample to A5 followed with addition of 2000µl diluent to A5 to make a 2% concentration of sample. Make serial dilutions of 1:2 (1000µl) from A5 to A4 … A3 to A2 and discard 1000µl from A2. This prepares a concentration range in increasing order from A1 to A5.
The Reagent vial was then removed from the freezer and reagent added to the Reagent Well as per the instructions. Add 10µl reagent from B1 to B5. The initial light intensity (Io) values were measured after pressing the Start button on the screen, by inserting cuvettes in Read well and pressing Read button every time. Sample transfers were made from A to B for all the concentrations and start the timer on software screen. The software will prompt for readings after 5 minutes and accordingly time-dependent intensity (It) values at 5 minutes can be measured. Similarly, intensities at different times, such as 5, 15, or 30 minutes, can be measured by inserting the times while starting the software.
After recording intensities or light measurements, the sample report appeared on the screen instantly displaying light intensity values, EC50 values, CI range, Toxicity units and graphs based on gamma and EC50 values. The Gamma values are based on pre- and post- intensities of light emissions by the bacteria. The typical ratio of 12ml distilled water to around 2.27g treated or untreated red mud were prepared and mixed in a shaker at 65°C for 2 days, in order to carry out accelerated simulated leaching of contaminants into an aquatic environment at room temperature. Then, the red mud material was separated by centrifugation, and the upper aqueous layer was analyzed with the Microtox® system.
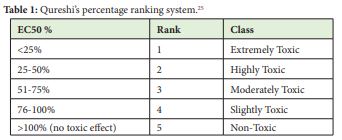
For toxicity ratings the Qureshi horizontal toxicity ranking system shown in Table 1 is followed [Qureshi25 and Bulich26 (Microbics Corporation). This means that for less than 25% of contaminant to reduce the level of bioluminescent bacteria by 50% corresponds to an extremely toxic contaminant. The other side of the range indicates that if greater than 100% of contaminant concentration reduces the bioluminescent bacteria to 50% of its original amount, then we have a nontoxic contaminant. These rankings, in turn, have a time-dependency based on the amount of time of exposure of the bioluminescent bacteria to the contaminant to result in its reduction to 50% level. In the Microtox® testing system, such exposure times are 5, 10 and 15 minutes.
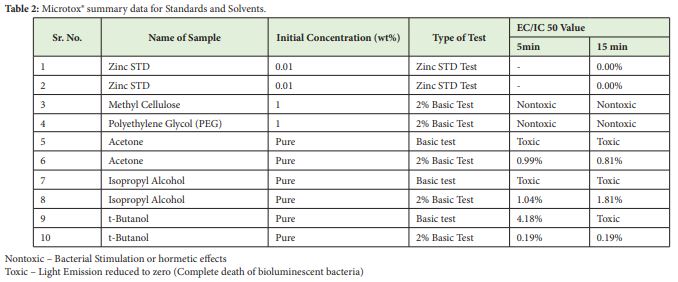
In Table 2 below, commercial chemicals were checked for comparability purposes. Before and during the test the recommended Zinc Sulfate hepta Hydrate standard test was performed according to the test protocol displayed by the Microtox® Omni software. The mean results were comparable to range 1.5mg/l-3mg/l.27,28 Further Acetone, Isopropyl Alcohol (IPA) and t-Butanol were tested for toxicity values considering their applications in oil spill dispersants as solvents for surfactants and crude oil. Before application of these solvents, an idea of toxicity range with Microtox® was important. The toxicity of all these solvents can be classified as toxic according to the ranking system indicated in Table 1. Acetone is less toxic than IPA and t-Butanol, but its effect as solvent in dispersant is very low since it can barely dissolve crude oil components. Similar effects are shown by IPA and t-Butanol and hence further toxic effects should be investigated. Also, there are other potential solvents as well as currently used solvents in the market for application in oil spill dispersion. Polyethylene Glycol (PEG) and Methyl Cellulose were tested as they are used in food industry and must show non-toxic effects. PEG is widely used in drug delivery system and biomedical applications.29 Methyl Cellulose also has similar applications. Both samples were tested at 1wt% in water and the test protocol followed was 2% Basic test. In both cases hormetic effects were observed implying the samples were nontoxic.
Polymeric surfactants prepared by Dr. Gerard T. Caneba, were tested for toxicity, aiming its applicability towards use in oil spill dispersion and oil recovery procedures. Along with that few commercially available surfactants were also tested as shown in Table 3. Table 4 further displays toxicity comparisons and classification of surfactants based on horizontal toxicity ranking system as tabulated previously in Table 3.
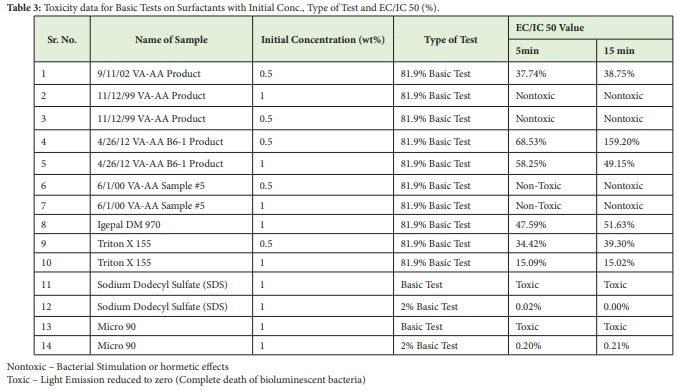
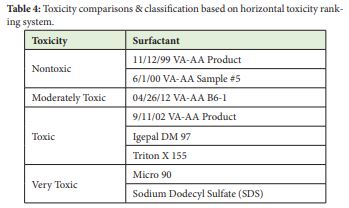
Horizontal ranking system analysis for toxicity, showed nontoxic effects by VA-AA surfactants, namely: 11/12/99 Product and 6/1/00 Sample #5 emulsions. In both cases there was increase in light intensities (It) at times 5 and 15min, compared to the intensities at time zero (I0). Aqueous VA-AA 4/26/12 B6-1 surfactant was moderately toxic with mean toxicity value between 51 and 75%. Further, 9/11/02 Surfactant, Igepal DM 970 and Triton X 155 were highly toxic with toxicity values between 25-50%. Commercial Detergent and Sodium Dodecyl Sulfate were ranked extremely toxic as expected.
In-house VA-AA and commercial surfactants mixed with crude oil were subjected to mechanically enhanced dispersion and tested for toxicity with the Microtox® equipment. Untreated crude oil and mechanically dispersed crude oil were also subjected for toxicity checks. Toxicity values for the aqueous crude oil/surfactant mixtures are shown in Table 5 below.
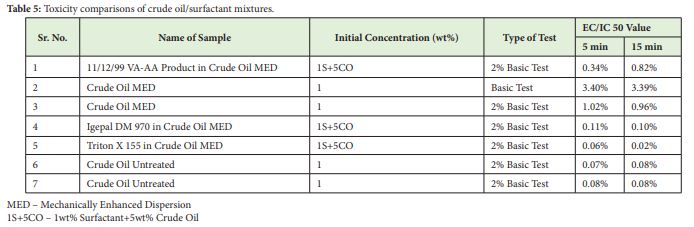
Untreated crude oil showed highest acute toxicity as compared to surfactant mixtures and dispersed crude oil only. It is evident that dispersion had reduced impacts on the luminescent bacteria. Further we look for addition of which surfactant had minimum effects. The readings suggest that 11/12/99 Product shows lesser toxicity compared to mixtures of commercial surfactants Igepal DM 970 and Triton X 155 dispersed with crude oil. The table shows toxicity comparisons of surfactant-crude oil mixtures tested for 2% Basic Tests, because of the limited solubility of crude oil in the aqueous dispersions.
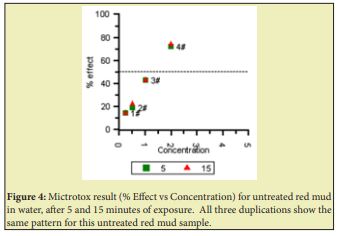
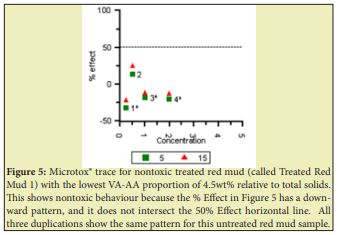
A plot of % Effect vs Concentration was obtained for untreated red mud (Figure 4), which indicates that red mud is toxic in water, because the points go through the 50% Effect horizontal line at relatively low concentration (EC50). Based on a collection of results, we also found that the lowest amount of VA-AA copolymer (6wt% AA relative to VA-AA solid) to treat red mud samples from pH=13 to pH=8 is 4.5wt% VA-AA relative to red mud and VA-AA (total solids basis), from the reactor fluid solution containing mostly azeotropic t-butanol-water (88.7wt% t-butanol) at 15wt% VA-AA copolymer or from solutions of VA-AA in either acetone, ethyl acetate, or THF. Treatment in this fashion resulted in non-toxic treated red mud though the Microtox® method, as shown in Figure 5. The reason is that the % Effect in Figure 5 has a downward pattern, and it does not intersect the 50% Effect horizontal line. The resulting material is a powder or a consolidated solid block that easily disintegrated into a coarse powder upon immersion in water. When partial pH treatment (down to pH value of 9) was employed for VA-AA incorporation of 3.3wt% relative to total solids, Microtox® analysis seemed to show borderline result.
Using of the Microtox® acute aquatic toxicity measurement method, the in-house vinyl acetate-acrylic acid (VA-AA) copolymer-based surfactants generated from the FRRPP process have been generally found to be nontoxic as self-emulsions in aqueous solutions. When the VA-AA copolymer-based surfactants are applied on liquid-liquid and liquid-solid interfaces as represented by crude oil-water and red mud-water mixtures, either reduced aquatic toxicities or toxicity elimination were realized.
None.
None.
The authors declare no conflict of interest.
- 1. Rosen MJ, Solash J. Factors affecting initial foam height in the Ross-Miles foam test. Journal of the American Oil Chemists’ Society. 1969;46(8):399–402.
- 2. Myers D. Surfactant Science and Technology. John Wiley & Sons, New York; 2006.
- 3. Caneba GT. Free-radical retrograde-precipitation polymerization (FRRPP): Novel concept, processes, materials, and energy aspects. Springer-Verlag, Heidelberg; 2010.
- 4. Caneba GT, Dar YL. Emulsion free-radical retrograde-precipitation polymerization (EFRRPP) and related topics. Springer-Verlag, Heidelberg; 2011.
- 5. Hemmer MJ, Barron MG, Greene RM. Comparative toxicity of eight oil dispersants, Louisiana sweet crude oil (LSC), and chemically dispersed LSC to two aquatic test species. Environ Toxicol Chem. 2011;30(10):2244–2252.
- 6. Ho KT, Kuhn A, Pelletier M, et al. Sediment toxicity assessment: comparison of standard and new testing designs. Arch Environ Contam Toxicol. 2000;39(4):462–468.
- 7. Bulich AA, Isenberg DL. Use of the luminescent bacterial system for the rapid assessment of aquatic toxicity. ISA Trans. 1981;20(1):29–33.
- 8. Jennings VL, Rayner Brandes MH, Bird DJ. Assessing chemical toxicity with the bioluminescent photobacterium (Vibrio fischeri): a comparison of three commercial systems. Water Res. 2001;35(14):3448–3456.
- 9. Whale GF, SRL. Potential applications of the Microtox® toxicity test within the offshore oil and gas industry. Society of Petroleum Engineers. 1994; p.687–698.
- 10. Johnson B. Microtox® acute toxicity test. In: Blaise C, Férard JF editors. Small-scale freshwater toxicity investigations. Springer, Netherlands; 2005. p.69–105.
- 11. Volpi Ghirardini A, Girardini M, Marchetto D, et al. Microtox solid phase test: Effect of diluent used in toxicity test. Ecotoxicol Environ Saf. 2009;72(3):851–861.
- 12. Alba P, Sánchez Fortún S, Alvarez Perez S, et al. Use of a microbial toxicity test (Microtox) to determine the toxigenicity of Aspergillus fumigatus strains isolated from different sources. Toxicon. 2009;53(7-8):729–733.
- 13. Fuller C, Bonner J, Page C, et al. Comparative toxicity of oil, dispersant, and oil plus dispersant to several marine species. Environ Toxicol Chem. 2004;23(12):2941–2949.
- 14. Abel PD. Toxicity of synthetic detergents to fish and aquatic invertebrates. Journal of Fish Biology. 1974;6(3):279–298.
- 15. Hall WS, Patoczka JB, Mirenda RJ, et al. Acute toxicity of industrial surfactants to Mysidopsis bahia. Arch Environ Contam Toxicol. 1989;18(5):765–772.
- 16. Marchetti R. The toxicity of nonyl phenol ethoxylate to the developmental stages of the rainbow trout, Salmo gairdnerii Richardson. Annals of Applied Biology. 1965;55(3):425–430.
- 17. Skidmore JF. Respiration and Osmoregulation in Rainbow Trout with Gills Damaged by Zinc Sulphate. Journal of Experimental Biology. 1970;52(2):481–494.
- 18. Swedmark M, Braaten B, Emanuelsson E, et al. Biological effects of surface active agents on marine animals. Marine Biology. 1971;9(3):183–201.
- 19. Layton AC, Gregory B, Schultz TW, et al. Validation of genetically engineered bioluminescent surfactant resistant bacteria as toxicity assessment tools. Ecotoxicol Environ Saf. 1999;43(2):222–228.
- 20. Environmental A.
- 21. George-Ares A, Clark JR, Biddinger GR, et al. Comparison of test methods and early toxicity characterization for five dispersants. Ecotoxicol Environ Saf, 1999;42(2):138–142.
- 22. Habashi F. A Short history of hydrometallurgy. Hydrometallurgy. 2005;79(1-2):15–22.
- 23. American Chemical Society. Production of Aluminum: The Hall-Héroult Process. National Historic Chemical Landmarks. Hall Process.
- 24. Ansar. Editor. Q., Microbiology methods manual, Alberta Enviroanmental Centre, Vegreville, Alberta; 1990. p. 437.
- 25. Bulich A. A practical and reliable method for monitoring the toxicity of aquatic samples. Proc Biochem. 1982;17:45–47.
- 26. Johnson B. Microtox® Acute Toxicity TestSmall-scale Freshwater Toxicity Investigations. Blaise C, Férard JF, editors. Springer Netherlands; 2005. p. 69-105.
- 27. Gaudet ID. Effect of Microtox Reagent Reconstitution Age on the Variability of Analytical Results from the Microtox Assay. Microbiology Research & Development, Alberta Research Council; 1999. p. 16-19.
- 28. Dutta A. Characterizaton of Polyethylene Glycol Hydrogels for Biomedical Applications, M.S, Thesis, Department of Chemical Engineering, Louisiana State University and Agricultural and Mechanical College; 2007. p.107.

