Objective: To know the effect of zinc supplementation on weight gain on premature newborns with very low birth weight.
Material and Methods: Randomized, blinded clinical trial, assigned to two groups: with zinc supplementation and placebo. Both with milk intake higher than 100ml/kg/day. We evaluated the absolute and relative growth speed, comorbidities, hospital stay and adverse reactions. We used Student´s t-distribution for the comparison of continuous variables, Mann-Whitney U test for nonparametric variables, X2 for categorical variables, and simple regression analysis for absolute and relative growth rate. P<0.05 was considered significant.
Results: We studied 56 patients, 28 in each group. The growth speed was higher in the supplemented group from the second week of treatment. The absolute and relative growth rate was 20.23±2.8g/day and 19.63±5.1g/kg/day in the zinc group and 17.13±3.5 g/day and 15.22±3.0g/kg/day in the placebo group, with p=0.0003 and 0.0001 respectively. The absolute and relative growth speed increased 3,104g/day (95%CI: 1,414-4,793) and 4,407g/kg/day (95% CI: 2,132-6,683) more in patients with zinc. There were no differences in morbidity or hospital stay and no adverse secondary reactions to the administration of zinc were detected.
Conclusions: The administration of zinc produces an increase in the growth rate of premature newborns with very low birth weight.
Keywords
Newborn, Premature, Zinc gluconate, Growth
Zinc is an essential nutrient that acts as an important cofactor of different enzymes that regulate the cellular growth and the hormonal levels.1 It is a micronutrient distributed all over the body that has critical effects for the child development. It participates in the cellular division and its growth. Also, it stimulates the absorption of electrolytes at the intestinal level, the neurotransmission, immune response, enzymatic catalysis, stabilization and functionality of membrane proteins, gene regulatory proteins and hormone receptors.2 Zinc is also required for DNA-binding proteins involved in the regulation of gene expression,3 so that its elimination produces hormone receptors that do not bind to DNA when activated by glucocorticoids or estrogens.4 With some degree of variability, as with many other nutrients, humans do not have functional or body stores of available zinc, except for full-term newborns, who can extract the accumulated hepatic zinc throughout the gestational period. This is not the case in premature newborns, in whom there is a reduction in available hepatic reserves during periods of reduced zinc intake.4 The zinc content in human milk varies considerably (0.7 to 1.6mg/L) and decreases over time. For example, colostrum contains 8–12mg/mL of zinc, while human milk, at 7 days of the newborn´s life, contains 3–6mg/L. Endogenous fecal zinc losses are estimated to range between 50 and 150ug/kg/day.5
Animal studies have shown that zinc deficiency decreases the expression of insulin-like growth factor 1 (IGF-1) and growth hormone receptor genes (GH) in the liver of growing rats.6 Other clinical studies report that zinc supplementation increases plasma IGF-1 levels in short statue children with or without zinc deficiency and improves growth and circulating IGF-1 levels in children with growth retardation. Thus, it is considered that the growth-enhancing action of zinc supplements is probably mediated by IGF-1.7 The oral dose of zinc in preterm newborns varies between 3 and 10mg/day.8 Zinc supplementation is much more beneficial in preterm infants with very low birth weight; that is, those weighing less than 1500 grams, regardless of their gestational age.9 Nutritional deficits, including micronutrient deficits, are nearly universal in very low birth weight infants, making them a prime target for early intervention with zinc supplementation.10 As an integral part of more than 200 enzyme systems in the human body, zinc is essential for normal child growth and development and its deficiency contributes greatly to poor growth.11
The aim of our study was to determine the effect of zinc gluconate supplementation on weight gain in preterm infants with very low birth weight. For this purpose, we use two indices basically associated with weight gain: absolute growth rate (AGR) and relative growth rate (RGR). The following formula was used to calculate the AGR: discharge weight in grams - birth weight in grams / days of life = g/day. An AGR larger than 20g/day is considered optimal. The RGR was calculated with the following formula: [weight at discharge in grams - birth weight in grams / days of life] / birth weight in kilograms = g/kg/day. The optimal RGR is considered from 15 g/kg/day.12
Aleatory, blinded, with randomized assignation clinical trial conducted in two groups of premature newborns (with zinc supplementation and without zinc supplementation) attended at the Mexico City Specialties Hospital Dr. Belisario Domínguez between September 19, 2019 and March 15, 2020. Inclusion criteria: premature newborns weighing less than 1500 grams, milk intake equal to or larger than 100ml/kg/day. Exclusion criteria: late establishment of the complete oral route (>15 days), necrotizing enterocolitis larger than Stage IA, and requirement for digestive surgery. Elimination criteria: patients who were not admitted to the Neonatal Intensive Care Unit (NICU), who were discharged early (<7 days), transferred to other hospital centers and who died during their stay in the service.
Before conducting the study, the nursing staff of the Neonatal Intensive Care Unit of our hospital was trained on the importance of an adequate supply of medications at the established times and adequate doses. We used zinc gluconate in 50mg tablets (z50®) that were pulverized and divided into 5mg sachets. The placebo (starch) was also packaged in 5mg sachets. This procedure was carried out in an establishment dedicated to the elaboration of didactic formulas. The sachets were stored at room temperature and without humidity. In selected patients, zinc gluconate was administered at a dose of 2mg/kg/day every 12 hours. The supplement (zinc or starch) was administered together with the intake of breast milk at 09:00 and 21:00. The nursing personnel were trained to fill the 5mg sachet in 5ml of bidistilled water and convert the indicated weight dose to the corresponding milliliters, which were administered together with the corresponding milk intake. The patients in both groups received exclusive breast milk by orogastric tube for 5 weeks and the people who diluted the powdered sachets with the milk did not know whether they were being given zinc or starch.
Upon admission to the NICU, general data, gestational age, birth weight, date and weight of study entry were recorded. During the patients' stay, the morbidities they presented were recorded and the following were considered the most frequent: bronchopulmonary dysplasia, late neonatal sepsis, pneumonia associated with health care, necrotizing enterocolitis, intraventricular hemorrhage and retinopathy of prematurity. In both groups, the average caloric intake received during the 5 weeks of follow-up with the administration of breast milk (caloric intake was calculated with the volume of milk expressed by the mother) and the tracing of the AGR and RGR was carried out weekly. At the patient's discharge, the total AGR and RGR, as well as the days of hospital stay, were recorded. Weight measurements were made by trained nursing personnel, always on the same weighing machine (Tronix 48O2D Pediatric/Infant Scale that reports weight from 10gr in the metric system and once the measurement is made it automatically returns to zero) and was based on the Manual of Anthropometric procedures provided by the United States Center for Disease Control (CDC).13
The study was approved by the Institution's Research Ethics Committee, with registration number: 501-010-23-19, and the informed consent of the newborn's mother or guardian was obtained.
With the antecedent that 80 premature newborns weighing less than 1500 grams are admitted to the NICU of our hospital annually, the participants were randomly assigned to one of the options. A proportion of 0.5 was considered for the two groups of newborns weighing less than 1500 grams and with a milk intake larger than 100ml/kg/day: group with administration of zinc gluconate and placebo group, with 95% confidence intervals (95%CI) and maximum estimation error of 0.072. The difference in proportions formula was used for a sampling without replacement with correction for finite population
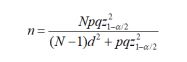
The sample size calculation was 57 newborns who were assigned 1:1 to each group. Normality tests were carried out through Kolmogorov-Smirnov and descriptive statistics were used: percentages, measures of central tendency and standard deviation and inferential statistics Student's T to compare the means of the quantitative variables in the two study groups, Mann's U Whitney for the contrast of the medians of the qualitative variables and Chi2 to establish independence between the categorical variables. Simple regression analysis was used, considering AGR and RGR as dependent variables and treatment as the only independent variable. In all cases, statistical significance was considered with p<0.05. The analysis was carried out in the statistical program SPSS version 20.
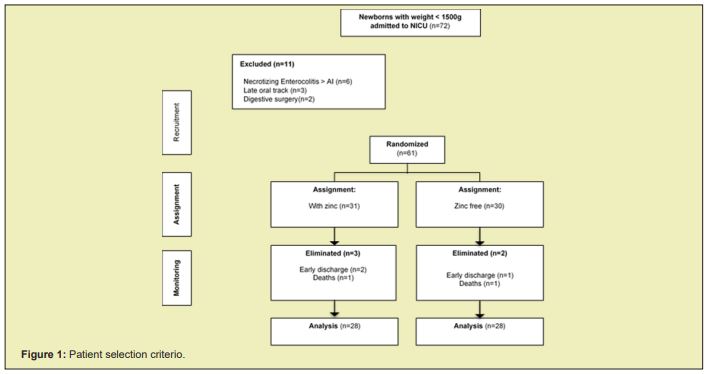
In the period between September 19, 2019 and March 15, 2020, 133 patients were admitted to the NICU of the Specialties Hospital Dr. Belisario Domínguez, of which 72 weighed less than 1500 grams. Of these, 6 were excluded due to necrotizing enterocolitis larger than grade 1A, 3 due to late establishment of the oral route and 2 due to digestive tract surgery (1 due to pyloric hypertrophy and another due to severe gastroesophageal reflux). 61 patients were randomly assigned to one of the groups: 31 patients in the group with zinc, of which 3 patients were eliminated, and 30 patients in the group without zinc supplementation, of which 2 patients were eliminated. The final analysis was carried out with 56 patients, 28 in each group. The patient selection criteria are shown in Figure 1.
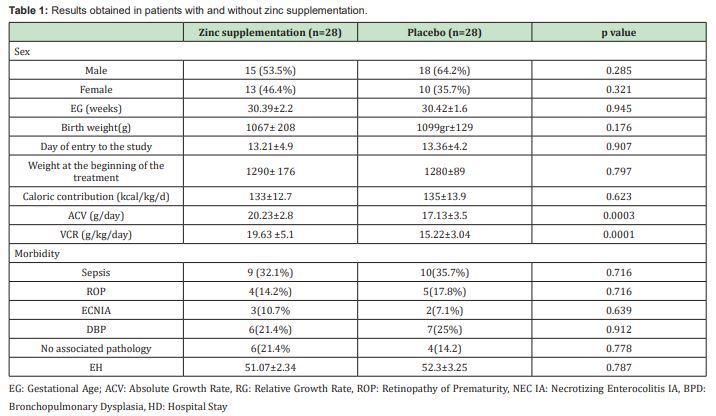
In the zinc supplementation group, 12 (42.9%) patients were male and 16 (57.1%) in the placebo group. The mean gestational age in the intervention group was 30.39±2.22 weeks with ranges between 25 and 35 weeks of gestation and in the placebo group, it was 30.43±1.60 weeks, with ranges between 28 and 35 weeks. When classifying the patients according to their gestational age into three groups (the first from 25 to 28 weeks, the second from 29 to 32 and the third from 33 to 35 weeks), the group of patients between 29 and 32 weeks was the one that included the largest number of study subjects. The average weight at birth was 1067±208 grams in patients who were administered zinc, with ranges between 550 and 1430 grams, and 1099±129 grams in patients without zinc administration, with ranges between 870 and 1430 grams. We did not find significant differences in gestational age (p=0.945) and birth weight (p=0.176) when comparing the groups. The mean postnatal age at entry to the study, and therefore at the start of follow-up, was 13.36±4.22 days, with ranges between 7 and 23 days for the placebo group. On the other hand, in the group with zinc supplementation, the mean postnatal age at entry to the study was 13.21±4.90 days with ranges between 7 and 22 days. We did not find a significant difference when comparing the age at entry to the study between both groups: p=0.907. The average weight at the time of starting the treatment was 1290±176 grams in the zinc supplemented group, and 1280±89 grams in the placebo group, with no significant difference between both groups: p=0.797. In the group supplemented with zinc, the average caloric intake during the five weeks of follow-up was 133±12.7 kcal/kg/day, and in the placebo group it was 135±13.9kcal/kg/day, with no significant difference between both groups: p=0.623.
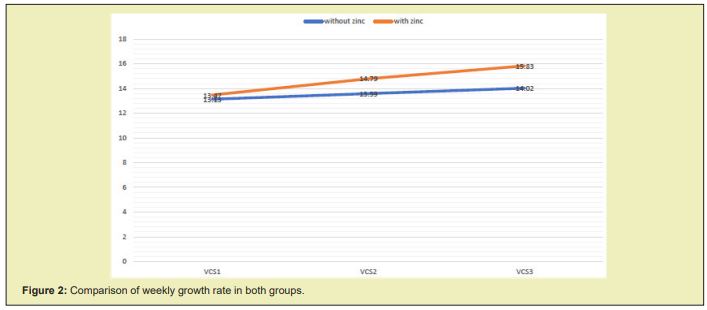
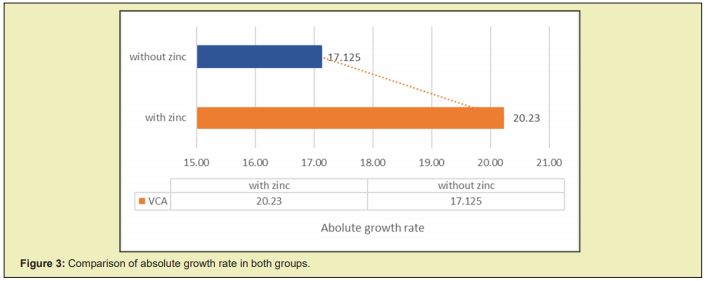
When we compare the weekly growth rate (g/day), we find a larger increase in the growth rate in the group supplemented with zinc from the second week after starting the management, as can be seen in Figure 2, in which a reduction in the parallelism of the growth curves between both groups is evidenced. The total AGR obtained at the end of the five weeks of follow-up was higher in the group supplemented with zinc, where a mean growth speed of 20.23±2.8g/day was observed compared to the placebo group with a mean growth speed of 17.13±3.5g/day, which was statistically significant: p=0.0003. Zinc supplementation produced an increase in the AGR of 3,107g/day more (95% CI: 1,414- 4,793) in relation to the non-supplemented patients, as observed in Figure 3.
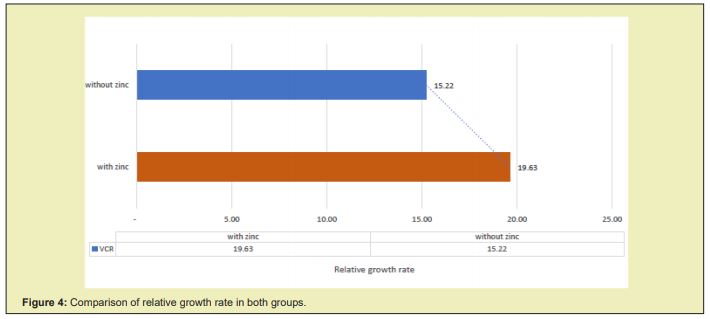
The total RGR obtained after five weeks of starting the treatment in the group supplemented with zinc was 19.63±5.1g/kg/day compared to the placebo group, which was 15.22±3.0g/Kg/day, which was statistically significant p=0.0001. Zinc supplementation produced an increase in RGR of 4,407g/kg/day more (95% CI: 2,132-6,683) in relation to non-supplemented patients, as evidenced in Figure 4.
When evaluating the associated comorbidities, 9 (32.1%) patients with sepsis were found in the group with zinc support and 10 (35.7%) in the group without zinc support, p=0.716. 4 (14.2%) patients with retinopathy of prematurity in the zinc group and 5 (17.8%) patients in the zinc-free group, p = 0.716. 3 patients (10.7%) with IA necrotizing enterocolitis in the zinc group and 2 (7.1%) among patients without zinc, p = 0.639. 6 (21.4%) patients with bronchopulmonary dysplasia in patients supplemented with zinc and 7 patients (25%) in patients without zinc, p = 0.912. Also, it was found that 6 (21.4%) patients in the zinc group did not present associated pathology and 4 (14.2%) in the group of patients without zinc intake, p=0.776. There was no significant difference when assessing mortality, since one death occurred in each group and no side effects were recorded after oral zinc administration. When comparing the average hospital stay in days, it was found that the patients supplemented with zinc had an average stay of 51.07±2.34 days compared to the non-supplemented patients, with an average stay of 52.3±3.25 days. When performing the statistical analysis, it was found that this difference was not significant, p=0.787. Table 1 summarizes the obtained results.
Adequate nutrition is especially useful in newborns with initial growth failure, such as premature newborns with very low birth weight, to prevent this from perpetuating and causing alterations in brain development that is particularly vulnerable to nutritional deficiencies in early stages of life.14 In this sense, early aggressive nutrition with high levels of calories and protein, as well as micronutrient supplementation to promote growth recovery is especially necessary in these patients.15 Some studies and systematic reviews of trials have reported an improvement in weight gain and linear growth in very low birth weight infants supplemented with zinc at different dosage regimens and for different durations.12,16,17 The relationship between IGF-1 and zinc was suggested many years ago by Ninh et al., and by Alves et al., who reported a significant increase in plasma levels of IGF-1 after 3 months of daily zinc supplementation of 5mg in children 6 to 9 years old.18,19 Osendarp et al., report that supplementation with zinc in patients 4 to 24 weeks of age only improved growth in children with reduced serum zinc levels and did not have this effect in patients without zinc deficiency.20 In our study, when comparing two groups of patients with gestational age, birth weight, weight at the beginning of treatment and similar caloric intake for 5 weeks, we found that the administration of zinc gluconate at 4mg/kg/day produces an increase in the growth speed that is manifested by a sustained weight increase from the second week of treatment. This became even more evident at the conclusion of the follow-up of these patients, when we found that the patients supplemented with zinc had a growth rate on average of 20.2g/day or 19.6g/kg/day compared to the non-supplemented patients who rose weight at an average rate of 17.1g/day or 15.2g/kg/day. Thus, the fact of supplementing zinc gluconate in premature newborns with very low birth weight improved the rate of weight gain by 3.1g/day and 4.4g/kg/day more in relation to patients who were not supplemented.
These findings are similar to the studies reported by Islam et al.,21 However, unlike these studies in which the weight gain was evident six weeks after starting the management, we found that the weight gain in the treated group was evidenced much earlier. This can be explained because in our study, the dose was doubled and, theoretically, as our patients were premature and with very low birth weight, they all had zinc deficiency. This dose of zinc would produce an increase in serum zinc levels, which in turn increases plasma levels of IGF-1, which is associated with an increase in weight and linear growth due to the well-recognized preferential action of zinc on the growth of cartilage and bone as reported by Yamaguchi et al.,22,23 As reported by Nakamura et al.,24 although zinc supplementation produces a significant increase in serum levels of IGF-1, it has no effect on the production of growth hormone (GH). However, when supplementing zinc in patients with deficiency of this micronutrient, another mechanism proposed for the increase in the growth rate of these patients is that the supplementation of this element improves or strengthens the binding of GH to its receptor. This could explain the effects of this element on weight gain in preterm infants with very low birth weight.
Unlike the reports by Osendarp et al.,20 in our study we found no differences in the morbidity of patients with and without zinc supplementation. This could be explained because their study was carried out in patients older than one month and who were not premature, unlike our study, in which we included premature babies with very low birth weight. This would explain the multiplicity of pathologies that these patients can present, which have different origins and are not only related to acute infections of the lower respiratory tract, where these authors report the usefulness of zinc by reducing the frequency of these. The adverse effects described for oral zinc supplementation, and which are more evident with doses bigger than 25mg/day,15 include vomiting, diarrhea, loss of appetite, and manifestations of copper and iron deficiency.16 No findings were found in any of the patients studied that are similar to those found by Imdad A et al.,25 and by Liu et al.26 We did not find a significant difference in the length of hospital stay, which could be explained by the fact that zinc has beneficial effects on increasing the growth rate of patients. However, it has little influence on various pathologies typical of premature patients such as bronchopulmonary dysplasia or retinopathy of prematurity, which are difficult to control and delay discharge, as reported by Díaz-Gómez et al.27
The oral administration of zinc gluconate is a useful therapy in patients with zinc deficiency, such as premature newborns with very low birth weight, in whom it produces a sustained increase in growth rate, evidenced from the second week after the start of treatment. Being a therapy with potential beneficial effects on the evolution of preterm newborns, our findings could contribute to generate new nutritional strategies in these patients that include zinc supplementation in patients with very low birth weight, reducing the possibility that these patients, with initial growth failure, have irreversible consequences for their health and development in later stages. Although in our study there was a tendency to discharge earlier in patients supplemented with zinc, this is a variable dependent on many other factors, so we consider that our results should be taken with caution. The limitations of our study are related to the small number of patients included and the lack of determination of serum zinc and IGF-1 levels, situations that could be remedied with future studies that continue this line of research.
None.
None.
Author declares that there is no conflict of interest.
- 1. McNall AD, Etherton TD, Fosmire GJ. The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic insulin-like growth factor 1 and growth hormone receptor genes. J Nutr. 1995;125:874e9.
- 2. Gibson RS, Hess SY, Hotz C, et al. Indicators of Zinc Status at the Population Level: A review of the evidence. Br J Nutr. 2008;99:S14–S23.
- 3. Hambidge KM, Krebs NF. Fetal Neonatal Physiol. Zinc in the Fetus and Neonate, Elsevier Saunders, USA: Philadelphia; 2004. p. 342–347.
- 4. Terrin G, Berni Canani R, Di Chiara M, et al. Zinc in early life: A key element in the fetus and preterm neonate. Nutrients. 2015;7:10427–10446.
- 5. Kennedy TS, Oakland MJ, Shaw RD. Growth patterns and nutritional factors associated with increased head circumference at 18 months in normally developing, low-birth-weight infants. J Am Diet Assoc. 1999;99(12): 1522-6.
- 6. Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998;68(2 Suppl):464S–469S.
- 7. Hoque A, Ali SMK. Role of Zinc in Low Birth Weight Neonates. Bang Med J. 2009;38(1):24–30.
- 8. Willoughby JL, Bowen CN. Zinc deficiency and toxicity in pediatric practice. Curr Opin Pediatr. 2014;26(5):579–584.
- 9. Patel A. Zinc supplementation for growth of preterm infants. Indian Pediatr. 2011;48(9):740.
- 10. Dekker LH, Villamor E. Zinc supplementation in children is not associated with decreases in hemoglobin concentrations. J Nutr. 2010;140(5):1035–1040.
- 11. Friel JK, Andrews WL, Matthew JD, et al. Zinc supplementation in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr. 1993;17(1):97–104.
- 12. Zamorano Jiménez, Clara Aurora J, Guzmán Bárcenas H, et al. Fernández-Carrocera. Pérdida de peso corporal y velocidad de crecimiento postnatal en recién nacidos menores de 1,500 gramos durante su estancia en un hospital de tercer nivel de atención. Perinatol Reprod Hum. 2012;26:187–193.
- 13. Cárdenas López Cristina, Haua Navarro Karime, Suverza Fernández Araceli, et al. Mediciones antropométricas en el neonato. Bol Med Hosp Infant Mex. 2005;62(3):214–224.
- 14. Cho JM, Kim JY, Yang HR. Effects of oral zinc supplementation on zinc status and catch-up growth during the first 2 years of life in children with non-organic failure to thrive born preterm and at term. Pediatr Neonatol. 2019;60(2):201–209.
- 15. Castillo Durán C, Weisstaub G. Zinc supplementation and growth of the fetus and low birth weight infant. J Nutr. 2003;133(5 Suppl 1):1494S–1507S.
- 16. Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477.
- 17. Ninh NX, Thissen JP, Collette L, et al. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-1) in growth-retarded Vietnamese children. Am J Clin Nutr. 1996;63(4):514–519.
- 18. Alves CX, Vale SH, Dantas MM, et al. Positive effects of zinc supplementation on growth, GH, IGF1, and IGFBP3 in eutrophic children. J Pediatr Endocrinol Metab. 2012;25(9-10):881–887.
- 19. Saskia JM Osendarp, Mathuram Santosham, Robert E Black. Efffec of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am J Clin Nutr. 2002;76(6):1401–1408.
- 20. Islam MN, Chowdhury MA, Siddika M, et al. Effect of oral zinc supplementation on the growth of preterm infants. Indian Pediatr. 2010;47(10):845–849.
- 21. Yamaguchi M, Inamoto K. Differential effect of calcium regulating hormones on bone metabolism in weanling rats orally administered zinc sulfate. Metabolism. 1986;35(11):1044–1047.
- 22. Yamaguchi M, Oishi H, Suketa Y. Zinc stimulation of bone protein synthesis in tissue culture. Biochem Pharmacol. 1988;37:4075–80.
- 23. Nakamura T, Nishiyama S, Futagoishi Suginohara Y, et al. Mild to moderate zinc deficiency in short children: effect of zinc supplementation on linear growth velocity. J Pediatr. 1993;123(1):65–69.
- 24. Imdad A, Bhutta ZA. Effect of preventive zinc supplementation on linear growth in children under 5 years of age in developing countries: a meta-analysis of studies for input to the lives saved tool. BMC Public Health. 2011;11Suppl 3(Suppl 3):S22.
- 25. Liu E, Pimpin L, Shulkin M, et al. Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients. 2018;10(3):377.
- 26. Díaz Gómez NM, Doménech E, Barroso F, et al. The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatrics. 2003;111(5 Pt 1):1002–1009.

