An 81-year-old male with past surgical history significant for right common femoral to posterior tibial artery bypass as well as aortic aneurysm repair using endograft presented with painful pulsatile right groin swelling. Ultrasound imaging revealed a fluid collection and concern for ruptured right common femoral artery aneurysm with expanding hematoma. The patient underwent prompt aneurysm excision with graft interposition complicated by wound dehiscence for which he subsequently underwent wound exploration, revision of proximal anastomosis and sartorius myoplasty. Our case illustrates a rare form of femoral artery rupture and operative management of a true common femoral artery aneurysm.
Keywords: Common femoral artery aneurysm, True femoral artery aneurysm, Femoral artery aneurysm rupture, Sartorius myoplasty
True femoral artery aneurysms (FAA) are extremely rare and defined as focal dilatations at least 1.5 times the diameter of the adjacent normal artery while involving all three layers of the arterial wall. In contrast, false aneurysms (pseudoaneurysms) are much more common and only contain one or two layers of the arterial wall.1,2 Of note, increased percutaneous vascular interventions have recently led to increased iatrogenic pseudoaneurysm formation.3 Type I FAAs involve only the common femoral artery (CFA), while type II FAA involve the origin of the profunda femoris artery (PFA). Complications associated with both true and false aneurysms include thrombosis, distal embolization and, very rarely, rupture.
Our patient is an 81-year-old male with a past medical history significant for end stage renal disease on hemodialysis, peripheral vascular disease, carotid artery stenosis, hypertension, and abdominal aortic aneurysm. Past surgical history included right CFA endarterectomy with right common femoral to posterior tibial artery vein bypass (October 11, 2011) due to limb ischemia and endograft repair of abdominal aortic aneurysm (AAA) with no known endoleak (Endurant, June 20, 2012). He is a current one to two pack-per-day smoker with no desire for cessation.
Unfortunately, patient completed endarterectomy with bypass at an outside facility and we are unable to retrieve those records. He underwent EVAR in 2012 with open technique. Of note, needle access was obtained into both right and left CFA with deployment of the main body graft (Endurant, 28x16x124) via right femoral access. No complications were noted postoperatively and patient underwent routine surveillance.
The patient was seen in clinic about one-month (June 12, 2019) prior during routine postoperative surveillance of his bypass with an arterial duplex in conjunction with abdominal ultrasound for aneurysm and carotid duplex for stenosis. The bypass duplex demonstrated a widely patent graft without significant stenosis, normal ABI, aneurysmal right CFA measuring 2.7cm (previously 2.3cm) with nonocclusive laminated thrombus as well as fluid collection measuring 5.9cm possible hematoma without evidence of abscess. In addition, aortic duplex imaging revealed a 3.4cm right common iliac artery aneurysm (previously 2.6cm). At this time patient was asymptomatic and decision was made to follow up in 6 months with repeat ultrasound and plans to proceed with elective repair in the setting of further aneurysmal growth.
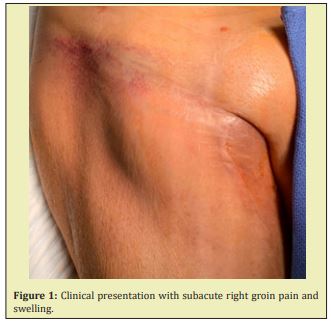
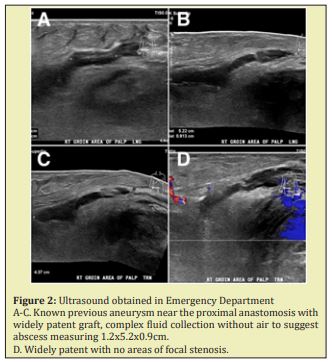
The patient presented to the ED complaining of a pulsatile painful mass in his right groin on June 29, 2019 (Figure 1). He reported that the groin pain and swelling of approximately two-to-three-day duration recent worsened in the last twenty-four hours. He was afebrile without any other subjective symptoms or complaints. Vital signs were all within normal limits. Physical examination was significant for a pulsatile mass in the right groin with diffuse ecchymosis. The bypass graft in the affected leg was easily palpable. Right popliteal and pedal pulses were also palpable. In addition, there was no pulsatile abdominal mass or evidence of left femoral or popliteal aneurysms. Ultrasound imaging revealed a complex fluid collection measuring 1.2x5.2x0.9cm without presence of air to suggest abscess. He was anemic with hemoglobin 7.6g/dL and no other laboratory abnormalities, including no leukocytosis. The patient was seen by the surgery team and scheduled for urgent repair with concern for a ruptured right CFAA and expanding hematoma Figure 2.
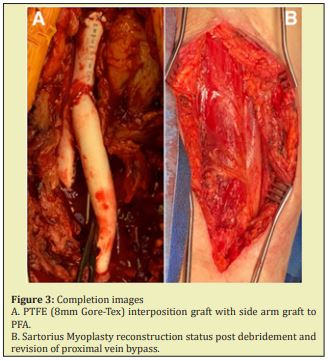
We performed a repair of the ruptured CFAA requiring PTFE interposition graft (8mm Gore-Tex) from the distal right external iliac artery to the PFA (end-to-end) as well as reimplantation of the saphenous vein bypass graft requiring PTFE graft (8mm Gore-Tex) from the common femoral graft to the origin of the saphenous vein graft. Bifurcated graft created with in situ technique. Recorded blood loss was 400cc. Pertinent intraoperative findings included obvious rupture of the CFAA without evidence of infection, with perforation located along posteromedial aspect of the native artery without involvement of the previous anastomosis. The origin of the vein graft was intact and there was no evidence of graft disruption causing the bleeding. The CFAA measured 3.5cm in diameter. No intraoperative cultures were sent due to lack of suspicion for infectious etiology.
The patient’s postoperative course was complicated by wound dehiscence and lymphatic drainage concerning for lymphocele. After attempted conservative management with wound care and antibiotics, we proceeded with groin exploration and sartorius myoplasty on July 31, 2019. We encountered copious amounts of foul-smelling necrotic tissue that required extensive debridement. Surgical wound cultures were sent. The anastomoses and grafts were skeletonized and all grafts and anastomoses were intact. The proximal vein and vein graft anastomoses, although patent, appeared to be infected and very thin. The decision was made to revise this segment with a short interposition graft (7 mm Gore-Tex) in an end-to-end fashion between the old PTFE and new PTFE graft. The distal end of the new PTFE was sutured in a end-to-side fashion to the previous vein bypass. To conclude, a sartorius flap myoplasty was performed for coverage with wound vac placement. Surgical cultures grew Vancomycin Resistant Enterococcus, Morganella, Enterobacter, and Peptostreptococcus. Patient recovered well postoperatively and discharged on postoperative day 8 with a four-week IV antibiotic regimen and close wound care follow up. Patient continues to reliably follow up for surveillance, however refuses smoking cessation recommendations.
CFAA rupture is a very rare occurrence (2-4%).4 As with other aneurysms, there is a strong male predilection for CFAA, and its incidence increases with advanced age.5 The average age is about seventy years-old and males are 5-16 times more likely to be affected than females. True FAA’s are associated with AAAs about 50-90% of the time and are commonly found bilaterally (25-50%). That said, the etiology and risk factors of CFAA are very similar to those of aneurysms of other vessels including hypertension, hyperlipidemia, smoking, male gender, old age, and previous vascular reconstruction. In the setting of previous reconstruction, anastomotic aneurysmal rupture usually occurs with infection about 60% of the time (not present in our case).
Patients are asymptomatic in about 30-40% of cases.6 Symptoms, when present, may include a pulsatile mass, pain, swelling, or sequelae of mass effect such nervewhich occurs in 30-40% of patients.7 Most commonly patients with femoral artery aneurysm present with claudication symptoms or ischemia secondary to distal embolization (65%).8 Very rarely, cases present with aneurysmal rupture (4%). Diagnosis is most often confirmed with ultrasound or computed tomography with angiography. Given the correlation between the presence of other peripheral arterial aneurysms, patients with CFAA should also be screened for abdominal aortic aneurysms with a duplex scan per USPTF guidelines.7,9 In addition, investigating presence of bilateral popliteal and contralateral femoral arteries is also critical as these aneurysms may be present concurrently. Recommended size for elective repair is 3.5cm, however symptomatic FAAs should be repaired regardless of size to minimize adverse events.10 The most common concerning complication is distal embolization. As expected, a strong correlation between the size of the aneurysm and the risk of complications has been demonstrated.11
In our patient, early recognition of rupture and surgical intervention was critical. Intraoperatively we noted a moderate degree of arterial wall calcification. There appeared to be a rupture of the CFA without evidence of infection. The origin of the vein graft was intact and there was no evidence of graft disruption causing the bleeding. In the setting of previous right sided CFA endarterectomy, progression of arterial disease and hemodialysis, as well as extensive smoking history, our patient was at risk for aneurysm formation and rupture. Although it is well accepted that aneurysms have about a 30% higher chance at developing aneurysms in patients with history of endarterectomy,12 our patient presented with native true common femoral artery rupture. We found a rare type 2 CFAA with the PFA arising from the aneurysmal sac itself. Given, the type of aneurysm and absence of infection, we resected the aneurysmal sac altogether and used a prosthetic interposition graft (PTFE) rather than a cryopreserved conduit. An end-to-end anastomosis was created from the distal external iliac artery to the PFA (Figure 3A). In addition, a second jump graft was placed from the new graft to the origin of the previous saphenous vein bypass graft. As discussed, the postoperative course was complicated by wound dehiscence and lymphatic drainage. After failed conservative management with wound care and antibiotics, we proceeded with groin exploration and sartorius myoplasty (Figure 3B).13 When performing this myoplasty, it is critical to dissect the lateral border of the muscle at its origin (anterior superior iliac spine) as the blood supply lies medial. After dissection, it is important to mobilize and divide through the tendinous portion and rotate the muscle from lateral to medial to cover femoral vessels. The patient completed the remainder of his postoperative course without any further complications.
This case report is noteworthy due to the rare encounter of a true CFAA rupture and its management. Two techniques outlined here include preserving the patency of the PFA and sartorius myoplasty reconstruction. Maintaining the patency of the PFA is critically important when possible because it serves as a collateral source of blood flow to the lower extremity via the lateral circumflex femoral artery.14 This becomes crucial for limb salvage in patients with chronic disease and potential SFA occlusion. Finally, the discussion behind sartorius myoplasty following vascular reconstructive surgery is important as the technique decreases the rate of wound infections and hastens groin closure thereby decreasing hospital stay and post-operative complications.
Patient contacted and verbal consent was obtained despite no patient identifiers being used.
Aubtin Saedi MSIV
None.
The authors have no disclosures or conflicts of interest.
- 1. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216–223.
- 2. Lawrence PF, Lorenzo Rivero S, Lyon JL. The incidence of iliac, femoral, and popliteal artery aneurysms in hospitalized patients. J Vasc Surg. 1995;22(4):409–415.
- 3. Niino T, Unosawa S, Kimura H. Ruptured common femoral artery aneurysm or abdominal aortic aneurysm?. Case Rep Surg. 2013;2013:306987.
- 4. Andrew M Cameron. Current Surgical Therapy. 13th ed. Elsevier; 2019.
- 5. Graham LM, Zelenock GB, Walter M Whitehouse. Clinical significance of arteriosclerotic femoral artery aneurysms. Arch Surg. 1980;115(4):502–507.
- 6. Piffaretti G. Twenty-year experience of femoral artery aneurysms. J Vasc Surg. 2011;53(5):1230–1236.
- 7. Tulla K, Qaja E. Femoral Aneurysm. Stat Pearls. Treasure Island (FL). 2020.
- 8. Dolapoglu A, Ertugay S, Posacioglu H. Staged approach for surgical management of a true femoral artery aneurysm combined with bilateral iliac artery aneurysms. SAGE Open Med Case Rep. 2017;5:2050313X17726911.
- 9. Savolainen H, Widmer MK, Heller G, et al. Common femoral artery uncommon aneurysms. Scand J Surg. 2003;92(3):203–205.
- 10. Rigdon EE, Monajjem N. Aneurysms of the superficial femoral artery: a report of two cases and review of the literature. J Vasc Surg. 1992;16(5):790–793.
- 11. Levi N, Schroeder TV. True and anastomotic femoral artery aneurysms: is the risk of rupture and thrombosis related to the size of the aneurysms?. Eur J Vasc Endovasc Surg. 1999;18(2):111–113.
- 12. Sigala F, Georgopoulos S, Sigalas K, et al. Femoral anastomotic aneurysms in the modern era: a reappraisal of a continuing challenge. Minerva Chir. 2006;61(2):95–101.
- 13. Petrasek PF, Kalman PG, Martin RD. Sartorius myoplasty for deep groin wounds following vascular reconstruction. Am J Surg. 1990;160(2):175–178.
- 14. Dardik H, Ibrahim IM, Sussman BC, et al. Remote profunda femoral bypass for limb salvage. Surg Gynecol Obstet. 1980;151(5):625–629.

