Cervicofacial necrotizing fasciitis is a rapidly evolving bacterial infection, which can be associated with multiple complications and poor health. However, there are few reported cases that are associated with Candida species, and the studies that report this indicate that it is found mainly in areas such as the pelvis, thorax and to a lesser degree in the arms and legs. The presence of Necrotizing Fasciitis in the cervicofacial region is very rare, which makes the present study relevant.
Material and Method: Descriptive, observational and retrospective study of Odontogenic Cervicofacial Necrotizing Fasciitis and the presence of Candida spp., Carried out during one year, in the Maxillofacial Surgery service of the Specialty Hospital, of the National Medical Center "La Raza" (IMSS). Patients older than 18 years, who were diagnosed with Necrotizing Cervicofacial Fasciitis of dental origin, were included in the study, reporting the presence of Candida. Treatment consisted of debridement of necrotic tissue, culture, dressings, intravenous antibiotics, and secondary surgical scrubs.
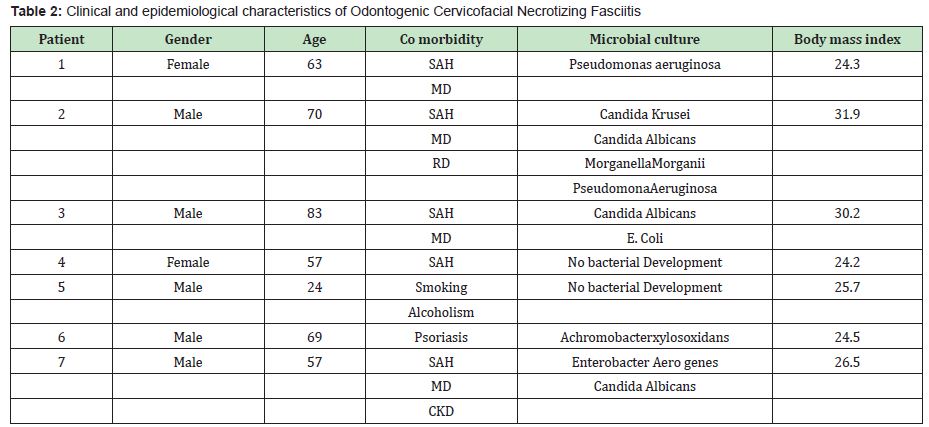
Results: In one year, 7 cases of Cervicofacial Necrotizing Fasciitis of dental origin were obtained, of which 3 patients had associated Candida species, the rest of the cultures were reported polymicrobial. The most associated comorbidities were uncontrolled diabetes mellitus and arterial hypertension, there was a predilection for the male sex, the median age was 63 years, the ranges (min: 24 and max: 83). In addition, it was found that the 3 patients who presented candida species in their cultures were overweight and their evolution was torpid.
Conclusion: It is important to perform cultures and biopsies to report the microbial species found in pathologies such as Necrotizing Fasciitis and to be able to provide better treatment specifically to the patient. Candida species do not usually appear associated with Necrotizing Fasciitis, which is why when other non-bacterial species are present, patients must undergo specific and individualized treatment to treat bacterial infections (necrotizing fasciitis) and in this case also the fungal (candida), remembering that the two act synergistically.
Keywords: Fasciitis, Necrotizing, Candida, Odontogenic, Infection, Cervicofacial
Abbreviations: SAH: Systemic Arterial Hypertension; MD: Mellitus Diabetes; RD: Respiratory Diseases (chronic obstretric pulmonary disease and pneumonia); CKD: Chronic Kidney Disease
Necrotizing fasciitis is an infection with a significant morbidity and mortality rate reported in the literature (ranging from 9% to 69%),1–4 the pathogenesis has not been fully specified, however it is known to be a multi bacterial infection in which you can present synergy with other opportunistic infections.1 The rapid spread of microorganisms through the facial planes leads to necrosis of the fascia and the overlying subcutaneous tissue.5 If the process continues, it spreads vertically towards the muscle.6 One of the main etiological factors mentioned in the literature is immune suppression, major or minor penetrating trauma, infections or cutaneous wounds with delayed healing, including open surgical wounds with a high risk of infection.7,8 The main pathogens associated with necrotizing fasciitis are bacterial, (necrotizing fasciitis can be divided by the type of bacterial biota that is present). Four types are reported in the literature,9–11 some authors only divide it into two large groups (polybacterial and monomicrobial).7,8 The complete classification is presented below in Table 1.
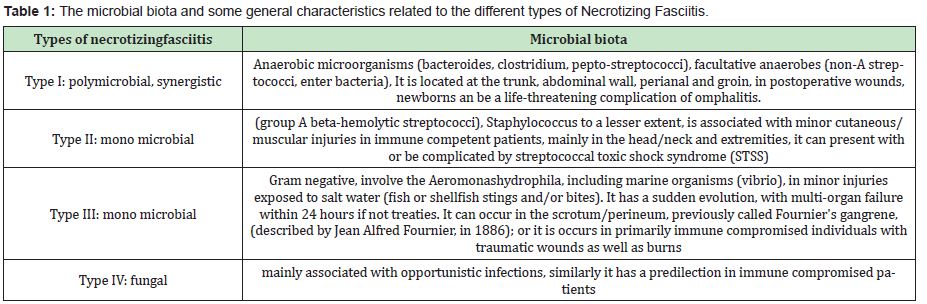
The most frequent types of Necrotizing Fasciitis are type I and type II, (polymicrobial mainly associated with anaerobic and monomicrobial bacteria related to Staphylococcus). However, there are reports that mention the presence of Candida species, which would link Necrotizing Fasciitis with a fungal etiology, which can occur opportunistically in the oral cavity or respiratory tract.7,12,13
Necrotizing fasciitis is a rapidly spreading infection, it occurs acutely, through the fascia and subcutaneous tissues. It can be located in different parts of the body (mainly in the pelvis, the lower extremities, the chest, followed by the upper extremities) and rarely occurs in the head and neck region.14,15 When necrotizing fasciitis occurs in the head and neck, it is called cervicofacial, and has two main subdivisions:
- a) Craniofacial if it is above the lower border of the mandible
- b) Cervical if it is below this border.15–18
Only 5% of the cases in this area are reported in the literature.18–20 Necrotizing fasciitis has been considered a polymicrobial disease, in which bacteria generate gases and end toxins that can act synergistically, leading to the development of the disease. The main associated species are: type I with anaerobes (among these bacteroides, clostridium, peptostreptococci), some facultative anaerobes (non-A streptococci), type II: is associated with group A beta-hemolytic streptococci (to a lesser extent C and G) and Staphylococcus aureus.9 the third type of Necrotizing Fasciitis is associated with Gram negatives, involves Aeromonashydrophila, including marine organisms (vibrio), and finally Type IV: fungal mainly associated with opportunistic infections (such as Candida spp.) (Table 1).10,11,21 The most affected patients are the immune suppressed, with uncontrolled diseases, wounds (open and/or deep) that do not heal, opportunistic infections, burns, trauma, among others.9 There are different case reports and studies that emphasize the presence of necrotizing fasciitis poly or monomicrobial (type I and II), however there are fewer studies that speak of necrotizing fasciitis type III (where it is found in cultures of Aeromonashydrophila) and type IV (fungal type, mainly associated with Candida species).7,12,13 The most common cause of cervicofacial necrotizing fasciitis is dental infections, the other etiologies include oropharyngeal infections (tonsillitis, peritonsillar abscess), insect bites, trauma, postoperative infection, long-term open wound infection and poor management, in addition to all these immune suppression or inadequate health status of the patients14,15,22–30 The management carried out by different authors and studies is based on intravenous antibiotics, initially it is empirical and then directed to the type of microorganisms found in the culture, in addition to multidisciplinary medical-surgical management, immediately to stop the progression of the disease (eliminating all necrotic tissue), ensuring that the wound margins are free of disease (necrosis) and preventing the further spread of necrotizing fasciitis. Different adjuvant therapies have been proposed, including negative pressure vacuum therapy, hyperbaric chamber/oxygen, local healing, debridement and surgical lavage, among others.11,17,26,28,29 On the other hand, Candida is a pathogen associated with a wide variety of diseases. It is a common colonizer of the oropharynx and intestines in normal individuals.29,31 Some species reported in studies on necrotizing fasciitis are Candida tropicalis and Candida Albicans,30–34 Candida albicans strains have been described as hyper virulent due to deterioration in patients with necrotizing fasciitis, however, no sufficient scientific evidence found about it.35–39 Among the main factors associated with the few reported cases of necrotizing fasciitis associated with Candida species (series of less than 20 patients [11 patients, 5 patients, 3 patients, etc.] or isolated cases are mentioned)12,13,40–42 They include: trauma, gunshot wounds, uncontrolled diabetes, significant immune suppression and transplant recipients (mainly kidney).12,13 Other species of Candida that can cause necrotizing fasciitis are: C. parapsilosis and C. glabrata.30,43,44 Few studies speak of the presence of Candida spp. in patients with necrotizing fasciitis and it is less common to find it in infections of the head and neck region, hence the interest in conducting this study, to know the type of micro biota that the patients presented and if any reported Candida in the culture.
Report the micro biota present in cases of odontogenic cervicofacial necrotizing fasciitis of the Maxillofacial Surgery service of the Specialty Hospital "La Raza", and report if any case with Candida spp.
An observational, descriptive and retrospective study was carried out; for one year (January-December 2014), in the Maxillofacial Surgery service of the Specialty Hospital “Dr. Antonio FragaMouret ”, National Medical Center" La Raza ", IMSS, during 2014. Reporting as isolated cases and frequencies. Including patients diagnosed with Cervicofacial Necrotizing Fasciitis of dental origin and reporting if any of them presented or developed Candida in culture, (remembering that it is rare to find it in this type of infection, unless it occurs opportunistically). The micro biota found was reported in microbiological cultures, as well as the characteristics presented by the patients. The management granted in the Maxillofacial Surgery service was with empirical intravenous antibiotics (beta-lactams and lincosamide), immediate radical surgery (removal of necrotic tissue), hospitalization and local cures on a scheduled basis (every 8 hours), performing mechanical work using sterile gauze and solution to thirds (hydrogen peroxide, sterile solution of sodium chloride 0.9% (w/v) in water and povidone iodine) and subsequently irrigating and cleaning the surgical bed only with physiological solution and sterile gauze; and finally placing an occlusive dressing over the wound. The inclusion criteria for this study were: patients of both genders (female or male), over 18 years of age, operated on in the Maxillofacial Surgery service of the Specialty Hospital of the National Medical Center “La Raza” during 2014 (from January to December), who had a diagnosis of Necrotizing Fasciitis secondary to dental abscess, whose cultures showed the presence of some species of Candida. The exclusion criteria were: Patients with a diagnosis of necrotizing fasciitis of origin other than dental, under 18 years of age. This study was carried out under the rules of the Local Ethics Committee of the Specialty Hospital of the National Medical Center "La Raza", taking care of the integrity and anonymity of all participants, it has signed informed consent from the patients included in the study, maintaining the data confidentially.
Data collection was carried out reporting the different variables to consider, such as: gender, age, co morbidities and culture. Reporting frequencies. Subsequently, the analysis of the results was carried out; the data management with SPSS version 15 was considered, with statistical analysis and graphical results.
In the Maxillofacial Surgery service of the Specialty Hospital “La Raza", in Mexico City, carried out during a year (January to December 2014), 7 cases of Cervicofacial Necrotizing Fasciitis of dental origin were obtained, of which 3 patients presented associated Candida species, the rest were polymicrobial, shown in Figure 1. the representative photos of these patients, observing in the surgical bed the presence of a whitish area especially in the center of the wounds, where the highest amount of Candida was reported (there were 2 different species of Candida: Candida Krusei and Candida Albicans) in all The presence of Candida Albicans was found in the culture and only one of the 3 patients had both species (C. Krusei and C. Albicans). Table 2 shows the different clinical and epidemiological characteristics according to the variables presented; reporting the presence of Odontogenic Cervicofacial Necrotizing Fasciitis in 7 patients predominantly male (5 men and 2 women), of which only 3 patients, all three were men, presented the association of Cervicofacial Necrotizing Fasciitis associated with Candida species, the which had torpid evolution and complications during their hospitalization; In addition, it is also appreciated that the most associated co morbidities were uncontrolled Diabetes Mellitus and Arterial Hypertension, however it is observed that the presence of respiratory diseases was found (Chronic Obstructive Pulmonary Disease and pneumonia), a patient with chronic kidney disease, with respect to age: the median 63 years and mode 57 years, the ranges (min: 24 and max: 83). In the same way, the rest of the results of the cultures are shown. A control was carried out on the Body Mass Index, finding that the 3 patients who presented Necrotizing Cervicofacial Fasciitis of Dental origin were overweight, reporting body mass indexes of 26.5, 30.2 and 31.9, the rest were within normal parameters, only one had 25.7. Hospital control, intravenous antibiotic management (with beta-lactam [cephalosporin or penicillin] and lincosamide [clindamycin]) was carried out for all patients. No patient was reported allergic to any medication, so this scheme was decided), all were intervened by the The same surgical team and all of them had a biopsy for culture in the same way (necrotic tissue with a healthy border), the histopathological study was carried out by the Central Laboratory of the Hospital "La Raza", and the results of the study were reported based on the initially stipulated.
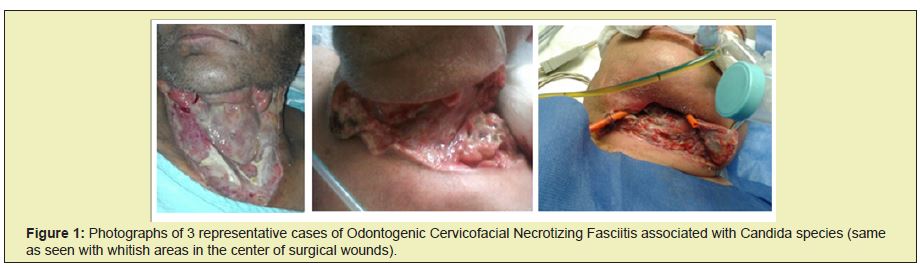
Necrotizing Fasciitis leads to progressive necrosis and worsening of the disease with systemic involvement, as has been reported in multiple studies45 in the present work presented it is shown again and coincidentally to the various authors that Necrotizing Fasciitis by itself is a pathology often of polymicrobial origin, which is also shown in this study, that it commonly affects the different superficial and deep layers of the body (skin, subcutaneous tissue, fat, muscle, including other structures such as vessels, nerves, organs). The diagnosis is based on the combination of clinical and histological characteristics of necrosis with the presented microbiology.46 It was found as a coincidence with other studies that the presence of Candida species associated with Necrotizing Fasciitis is rare but draws attention because they are two infections that occur at the same time in patients who are mainly immunocompromised, it can occur recurrently or as infection added to necrotizing fasciitis opportunistically47 Calderone, Fonzi, Niewerth and Korting among other authors48,49 mention that Necrotizing Fasciitis has a mechanism with end toxins and bacteria that generate gases (for example, clostridial species) and it is known that Candida acts in a similar way causing diseases where gas is produced, obtaining the hypothesis that this gas is a by-product of fermentation and for the virulence of the candida to be significant is supported by determining factors in human infections such as the presence of adhesins, phospholipases, morphogenic changes, phenotypic aspartyl proteinases switched and secreted12,48,49 Of the aforementioned factors, Adhesins are bimolecular that promote the adherence of Candida albicans to those of the patient.48 mentioning that the variants or mutations of the candida Albicans do not present said molecules so they are less or null virulent. For years it has been mentioned that Candida grows as single-celled yeast as filamentous pseudohypha and hyphal forms. Which are found in the invaded tissue, generating morphogenetic changes, which was corroborated with histopathological studies and cultures.12,49 Fontes et al.50 mention that when necrotizing fasciitis related to an infection by Candida species occurs, the patients at greatest risk of developing this form of the disease are those who are immune suppressed, mainly with poorly controlled diabetes or peripheral vascular disease.50 Which coincides with the study presented, given that the 4 patients who presented with a finding of a Candida species had Systemic Arterial Hypertension and Diabetes, one of them also had other pathological agents that are associated with serious infections, such as presented in the Morganella Morganii culture the patient presented with pneumonia of torpid evolution and worsening of the general state of health of the patient with rapid dissemination to the mediastinum. Eisen and Abbasi mention that within the immune suppressions associated with Necrotizing Fasciitis associated with Candida, there are patients with kidney disease or who have had a kidney transplant and, like said authors, in this study one of the patients had chronic kidney disease and presented in his culture Candida12,13 Therefore, this article coincides with the study carried out by Brook et al.51 O Atallah et.al among others52 mention that necrotizing- type infections that have been associated with the presence of Candida species act synergistically, which is still under study by various authors. There are not enough cases that report the presence of Candida Albicans in the cervicofacial region and associated with dental origin.44,53,54
Necrotizing Fasciitis has a rapid evolution and complications such as systemic and structural involvement (deep and superficial). Few studies of cervicofacial necrotizing fasciitis are reported in the literature, and even fewer cases are related to odontogenic infections. Most of the articles mention the presence of Candida spp. (Albicans) in patients with Necrotizing Fasciitis secondarily and located in different parts of the body (mainly pelvis and thorax) but they are not common in the head and neck region; which is important since the cases presented in this article are found in the cervicofacial and craniofacial region. It is also mentioned that the presence of Candida is uncommon in cases of Necrotizing Fasciitis, which makes the present study important since it is not common to find type IV Necrotizing Fasciitis (classification by microorganisms) in the head and neck. Generally, various species of microorganisms are present (mainly bacteria). The surgical medical treatment is the same (regardless of the type of necrotizing fasciitis I-IV). One of the difficulties found in this work is that a statistically strong association could hardly exist, due to the type of sample presented. But this study can help as a basis for subsequent studies to continue with the quantitative analysis and have a larger sample. On the other hand, it is important to take into account the symptoms reported by the patient and give them the benefit of the doubt, since it should be considered as suspicion in the evaluation of patients, especially if they are immune suppressed (due to the greater susceptibility to contracting infections), the search to Find any relationship between necrotizing fasciitis and the presence of Candida or other microorganisms can help to continue the search for more scientific evidence and larger case series or multicenter studies to investigate and propose better management and treatment options, specifically aimed at these types of infections.
We appreciate the support provided by the Editorial Committee and editor-in-chief, for their understanding and help in presenting this research study. We have not received financing nor do we have sponsors to carry out this study.
The authors have declared no competing interest. The study has not been funded by any institution. We confirm all relevant ethical guidelines have been followed.
None.
- 1. McHenry C, Piotrowski J, Petinic D, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221(5):558–563.
- 2. Fustes A, Gutierrez P, Duran C, et al. Necrotizing fasciitis: Report of 39 pediatric cases. Arch Dermatol. 2002;138(7):893–899.
- 3. Wagner J, Prevel C, Elluru R. Histoplasma capsulatum necrotizing myofasciitis of the upper extremeity. Ann PlastSurg. 1996;36(3):330– 333.
- 4. Korhonen K. Hyperbaric oxygen therapy in acute necrotizing infections. With a special reference to the effects on tissue gas tensions. Ann Chir Gynaecol. 2000;89(Suppl 214):7–36.
- 5. Rea W, Wyrick W. Necrotizing fasciitis. Ann Surg. 1970;172(6):957–964.
- 6. Childers B, Potyondy L, Nachriener R, et al. Necrotising fasciitis: A fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002;68(2):109–116.
- 7. Bartram L, Aaron J. Fungal necrotizing skin and soft tissue infections. Current Fungal Infection Reports. 2019;146–156.
- 8. Stevens D, Bryant S. Necrotizing soft-tissue infections. N Engl J Med. 2017;377(23):2253–2265.
- 9. Parra P, Pérez S, PatinoM, et al. Updateonnecrotizingfasciitis. Semin Fund Esp Reumatol. 2012;13(2):41–48.
- 10. Wong C, Wang Y. The diagnosis of necrotizing fasciitis. Curr Opin Infect Dis, 2005;18(2):101–106.
- 11. Lancerotto L, Tocco I, Salmaso R, et al. Necrotizing fasciitis: Classification, diagnosis, and management. J Trauma Acute Care Surg. 2012;72(3):560– 566.
- 12. Eisen D, Brown E. Necrotizing Fasciitis Following a Motor Vehicle Accident with Candida Species As the Sole Organisms. Can J Plast Surg. 2004;12(1):43–46.
- 13. Abbasi Z, Inam H, Das S, et al. Fungal Cervical Abscess Complicated by Necrotizing Fasciitis Leading to Descending Necrotizing Mediastinitis: A Case Report. Cureus. 2019;11(8):e5369.
- 14. Mohammedi I, Ceruse P, Duperret S, et al. Cervical necrotizing fasciitis: 10 years’ experience at a single institution. Intensive Care Med. 1999;25(8):829–834.
- 15. Oguz H, Yilmaz MS. Diagnosis and management of necrotizing fasciitis of the head and neck. Curr Infect Dis Rep. 2012;14(2):161–165.
- 16. Lanisnik B, Cizmarevic B. Necrotizing fasciitis of the head and neck: 34 cases of a single institution experience. Eur Arch Otorhinolaryngol. 2010;267(3):415–421.
- 17. Juárez A, Juárez C, Juárez D. Craniofacial Fasciitis Secondary to Odontogenic Infection. J Dental Sci Res Rep. 2020;2(3):1–4.
- 18. Ellis S, van Orman-ER, Hatch-BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134(2):293–299.
- 19. Endorf F, Cancio L, KleinM. Necrotizing soft-tissue infections: Clinical guidelines. J Burn Care Res. 2009;30(5):769–775.
- 20. Yoder B, Deeb Z. Necrotizing Fasciitis of the Face: Case Report and Review of the Literature. Otolaryngol Head Neck Surg. 2004;2:288–294.
- 21. Juárez A, López F, Juárez D, et al. Cervicofacial Necrotizing Fasciitis and Its Relationship with Aeromona Hydrophila. J Dent Oral Sci. 2020;2(4):1– 12.
- 22. Panda N, Simhadri S, Sridhara S. Cervicofacial necrotizing fasciitis: can we expect a favourable outcome? J LaryngolOtol. 2004;118(10):771– 777.
- 23. Shaikh N, Ummunissa F, Hanssen Y, et al. Hospital epidemiology of emergent cervical necrotizing fasciitis. J Emerg Trauma Shock. 2010;3(2):123–125.
- 24. Thakur J, Verma N, Thakur A, et al. Necrotizing cervical fasciitis: prognosis based on a new grading system. Ear Nose Throat J. 2013;92(3):149–152.
- 25. Mathieu D, Neviere R, Teillon C, et al. Cervical necrotizing fasciitis: clinical manifestations and management. Clin Infect Dis. 1995;21(1):51–56.
- 26. Flanagan C, Daramola O, Maisel R, et al. Surgical debridement and adjunctive hyperbaric oxygen in cervical necrotizing fasciitis. Otolaryngol Head Neck Surg. 2009;140(5):730–734.
- 27. Tung-Yiu W, Jehn-Shyun H, Ching-Hung C, et al. Cervical necrotizing fasciitis of odontogenic origin: a report of 11cases. J Oral Maxillofac Surg. 2000;58(12):1347–1352.
- 28. Bakshi J, Virk R, Jain A, et al. Cervical necrotizing fasciitis: our experience with 11 cases and our technique for surgical debridement. Ear Nose Throat J. 2010;89(2):84–86.
- 29. Juárez A, López F, Juárez D, et al. Frequency of cervicofacial necrotizing fasciitis secondary to odontogenic abscess. Dental Res Manag. 2020;4:46–51.
- 30. Zhang M, Chelnis J, Mawn L. Periorbital necrotizing fasciitis secondary to Candida parapsilosis and Streptococcus pyogenes. Ophthalmic Plast Reconstr Surg. 2017;33(3S Suppl 1):S31–S33.
- 31. Rath S, Kar S, Sahu S, et al. Fungal periorbital necrotizing fasciitis in an immunocompetent adult. Ophthalmic Plast Reconstr Surg. 2009;25(4):334–335.
- 32. Fotos P, Lilly J. Clinical management of oral and perioral candidiosis. Dermatol Clin. 1996;14(2):273–280.
- 33. Lacour M, Zunder T, Huber R, et al. The pathogenetic significance of intestinal Candida colonization–A systematic review from an interdisciplinary and environmental medical point of view. Int J Hyg Environ Health. 2002;205(4):257–268.
- 34. Hazen K. New and emerging yeast pathogens. Clin Microbiol Rev. 1995;8(4):462–478.
- 35. Tams R, Cassilly C, Anaokar S, et al. Overproduction of phospholipids by the kennedy pathway leads to hypervirulence in Candida albicans. Front Microbiol. 2019;10:86.
- 36. Kumar R, Saraswat D, Tati S, et al. Novel aggregation properties of Candida albicans secreted aspartyl proteinase sap6 mediate virulence in oral Candidiasis. Infect Immun. 2015;83(7):2614–2626.
- 37. Wartenberg A, Linde J, Martin R, et al. Microevolution of Candida albicans in macrophages restores filamentation in a nonfilamentous mutant. PLoS Genet. 2014;10(12):e1004824.
- 38. Yu S, Chang Y, Chen Y. Deletion of Ada2 increases antifungal drug susceptibility and virulence in candida glabrata. Antimicrob Agents Chemother. 2018;62(3):e01924–17.
- 39. Gank K, Yeaman M, Kojima S, et al. SSD1 is integral to host defense peptide resistance in Candida albicans. Eukaryot Cell. 2008;7(8):1318– 1327.
- 40. Buchanan O, Mast B, Lottenberg L, et al. Candida albicans necrotizing soft tissue infection a case report and literature review of fungal necrotizing soft tissue infections. Ann Plast Surg. 2013;70(6):739–741.
- 41. Perkins T, Bieniek J, Sumfest J. et al. Solitary Candida albicans infection causing fournier gangrene and review of fungal etiologies. Rev Urol. 2014;16(2):95–98.
- 42. Wai P, Ewing C, Johnson L, et al. Candida Fasciitis Following Renal Transplantation. Transplantation. 2001;72(3):477–479.
- 43. Loulergue P, Mahe V, Bougnoux M, et al. Fournier’s gangrene due to Candida glabrata. Med Mycol. 2008;46(2):171–173.
- 44. Lee, W Choi, JY Chan. Candida parapsilosis associated with cervical necrotizing fasciitis and descending mediastinitis. J Surg Case Rep. 2017;2017(4):rjx065.
- 45. Cainzos M, Gonzalez F. Necrotizing soft tissue infections. Curr Opin Crit Care. 2007;13(4):433–439.
- 46. Anaya D, Dellinger E. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705–710.
- 47. Bilton B, Zibari G, McMilan R, et al. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study/ discussion. Am Surg. 1998;64(5):397–400.
- 48. Calderone R, Fonzi W. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327–334.
- 49. Niewerth M, Korting C. Phospholipases of Candida albicans. Mycoses. 2000;44(9-10):361–367.
- 50. Fontes R, Ogilvie C, Miclau T. Necrotizing soft-tissue infections. J Am AcadOrthop Surg. 2000;8(3):151–158.
- 51. Brook I, Frazier EH. Clinical and microbiological features of necrotising fasciitis. J Clin Microbiol. 1995;33(9):2382–2387.
- 52. Atallah N, et al. Candida albicans necrotizing fasciitis following elective surgery. Med Mycol Case Rep. 2020;28:39–41.
- 53. Bayetto K, Cheng A, Sambrook P. Necrotizing fasciitis as a complication of odontogenic infection: a review of management and case series. Australian Dental Journal. 2017;62:317–322.
- 54. Smeekens S, van de Veerdonk F, Kullberg B, et al. Genetic susceptibility to Candida infections. EMBO Mol Med. 2013;5(6):805–813.

