Brown adipose tissue (BAT) plays a significant role in the expression of non-shivering thermogenesis in response to perturbations in diet and environment in man and animals. The SHR/N-cp rat is an animal model of obesity and T2DM and has been reported to exhibit an impaired thermogenic response to parameters of diet and environment. Groups of lean and obese male SHR/N-cp rats were maintained in hanging wire-bottomed steel cages and fed a nutritionally complete diet containing 54% CHO, 22% protein,16.5% mixed fats, and 4.5% essential fiber, plus vitamins, minerals, and essential micronutrients from 1 to 9 months of age. Measures of body weight were monitored and 24-hour urinary vanil mandelic acid (VMA) were determined at the end of the study. Animals were sacrificed by decapitation and the Interscapular BAT depots excised in their entirety for measures of adipocyte size, number, and lipid content. Bode weights, net weight gain and relative adiposity of Obese was significantly greater than their lean littermates throughout the study. Urinary VMA of lean > obese rats. The IBAT weight and IBAT weight: Body weight of obese >> lean. IBAT cell number, cell lipid content and % lipid of IBAT tissues of obese >> lean of obese rats. The results of this study indicate that while the development of IBAT mass and cellularity becomes exaggerated in the obese-diabetic animals, the superimposition of the T2DM stigmata including likely insulin resistance may further compromise the capacity of the obese diabetic animals to fully express BAT-mediated contributions to NST.
The role of brown adipose tissue (BAT) and sympathetic neural system (SNS) activity in the expression a development of mechanisms of non-shivering thermogenesis in rodents and other mammals is well established. The primary function of BAT is deemed to convert energy derived from food into heat that may be dissipated in homeothermy and in the maintenance of body temperature.1 BAT is especially important in the newborn of several mammalian species, and in maintaining body temperature following cold exposure and in cold environments. BAT can become activated via signals initiated from the hypothalamus, and transmitted to the BAT directly via sympathetic neurons, which bring about the release of the neurohormone norepinephrine. BAT contains stereo specific specialized β3 adrenergic adreno receptors, unique to brown adipocytes which bring about the activation of the thermogenic activity and the hydrolysis of high energy phosphate bonds, each of which can release approximately 7.2kcals of energy in the form of heat. The uncoupling protein-1 (UCP1) that mediates the activation of BAT thermogenesis is unique to brown adipose tissue of mammalian organisms.1,2 Himms-Hagen, Rothwell and Stock, and others have established the role and biochemical mechanisms of BAT-mediated thermogenesis in rats and humans, and Tulp et al established the effects of early over nutrition and of nutritional manipulation on hyperplasia and hypertrophy of brown adipose tissue in young Sprague-Dawley rats when overfed or fed calorically adequate but protein restricted diets from weaning to adolescence.1-5 Once formed, the increased BAT mass and cellularity persisted well into adulthood in those studies.6 In those early studies, the BAT mass and cellularity increased by more than 2-fold during the early post weaning period. The thermogenic responses to norepinephrine occurred normally in proportion to the increased BAT mass in metabolically normal lean rats, but catecholamine-stimulated responses in obese and obese diabetic animals demonstrating various degrees of insulin resistance have been shown to become impaired by early adulthood. Marette et al demonstrated that insulin sensitivity Is an essential element in the catecholamine mediated expression of BAT thermogenesis7 and Tulp et all showed that the resting, and norepinephrine stimulated thermogenesis in obese diabetic (T2DM) rats of several strains demonstrated impaired thermogenic responses to diet and environment.7-10 The peripheral uptake of glucose, and insulin dependent process in muscle and adipose tissue has also been linked to thermogenesis in peripheral tissues.8-10 Himms-Hagen and others have suggested that a fundamental role of BAT thermogenesis in the early life of a mammalian species is to generate necessary heat ostensibly to contribute to temperature regulation in the newborn and early life stages, where the surface area to metabolic mass is greater in proportion than occurs later in life.1-3,9-10 In addition, the added benefit of extra BAT could also facilitate biochemical mechanisms that contribute to energy balance by converting excess energy to heat, thereby minimizing the effects of the excess caloric impact on fat accretion in adipose tissue. The development of the obese (-cp) phenotype occurs via an epigenetic expression, commencing soon after weaning in genetically obese rodents.8 The cellular characterization of brown adipose tissue in lean animals has been described but the BAT characteristics in obese T2DM rats of this strain have not heretofore been described. The early and chronic hyperphagia common among obese and obese T2DM strains in concert with enhanced caloric efficiency are suggestive entities as contributing predisposing factors in induction of the hypertrophy and hyperplasia in a physiologic attempt to attenuate the potentially adverse impact of the early onset hyperphagiaon development of comorbidities via enhanced energy deposition and storage as fat, which should it become extreme may result in dire outcomes with regards to health and longevity in man and animals.11,12
The activation of non shivering thermogenesis in the obese-diabetic (T2DM) SHR/Ntul//-cp rat has been shown to be impaired, at least in part secondary to the effects of insulin resistance in brown adipose tissue (BAT), where sensitivity to insulin actions is essential for expression of thermogenesis in isolated brown adipocytes.7,9-10 Impaired parameters of thermoregulation and energy expenditure facilitates an efficiency and ease of weight gain, contributing to the epigenetic expression, development, and maintenance of an obese phenotype.11 The magnitude of insulin resistance is further magnified in the presence of T2DM, which likely further impairs unobstructed thermogenic activity. BAT is abundantly innervated by the sympathetic division of the autonomic nervous system and is increased in lean animals during periods of caloric over nutrition, including carbohydrate feeding.1-3 As a result of the increased SNS activity in response to diet and environment, urinary excretion of norepinephrine metabolites via conversion to vanilmandelic acid becomes increased in proportion to the SNS activity and appears in the urine.13-14 Glycosuria can contribute to an osmotic diuresis, resulting in increased volumes of urine especially in uncontrolled T2DM, while the osmotic impact of the urinary catecholamine metabolites is relatively minor in nature owing to the smaller osmolar contributions to final urine volumes.14
The incidence of overweight and obese conditions has now become prevalent in numerous developed societies in concert with advances in nutritional science and technology, and where it now borders on becoming epidemic, in spite of numerous advances in nutrition knowledge and practice.15 The overall impact on society of obesity and its related comorbidities including NIDDM, cardiovascular disease, and others has not placed an enormous burden on the economy due to lost productivity, and on the health care resources of developed societies.
The development of the SHR/N-cp rats is depicted in Figure 1 below andshows that the -cp (corpulent) trait originally derived from the Koletsky rat was bred into the Spontaneously Hypertensive rat (SHR) rat at the VRB, NIH by Hansen as part of an ongoing project to develop a congenic and SPF animal model for investigation of obesity and its comorbidities.16,17 The current SHR/Ntul//-cp rat strain was established from original breeding pairs derived from the 12th backcross of the original stock. The -cp trait is transmitted as an autosomal recessive trait and becomes evident in one quarter of the offspring of heterozygous breeding pairs by 6 weeks of age, and early stigmata of T2DM appears spontaneously by 8 to 10 weeks of age among the obese offspring.16
Groups of 4–5-week-oldmale lean and obese littermates of SHR/Ntul-cp rats were obtained from the Drexel colony, originally developed from breeding pairs obtained from the Veterinary Resources Branch of the NIH in Bethesda, MD, USA. Rats were fed a standard diet consisting of 54% CHO as cornstarch, 22% protein, 16.5% mixed fats, 4.5% crude fiber plus essential vitamins and minerals from four weeks until 9 months of age. Animals were housed in steel, hanging steel cages at 22°C and 50% RH on a reverse light cycle. Measures of body weight were obtained periodically throughout the study. Rats were sacrificed by decapitation, fasting bloods collected for measures of glucose and insulin concentration, and the Interscapular (IBAT), Dorsal, Retroperitoneal and Epididymal fat depots dissected in their entirety. Urine was collected in 1 ml of 6N HCL to preserve catecholamine metabolites over 24-hour periods and urine volumes recorded.18 Sections of IBAT of approximately 50 mg each were weighed to the nearest 0.005 g, and double-fixed with formalin and osmium tetroxide, and the particle size and volume determined on a Coulter particle counter.4 In WAT depots, 50 mg sections of tissue were collected and fixed directly in OsO4 solution to fix and immobilize the lipid content, and counted in a Coulter particle counter.4 Tissue lipid content was determined gravimetrically as described by Hirsch and Gallian.19 Urine glucose was det1ermined qualitatively, and urinary vanilmandelic acid as a reflection of sympathetic activity measured as outlined previously.20,21 Tissue depots were dissected in their entirety and weighed on an Ohaus electronic balance to the nearest 0.1gram within one minute, and before significant dehydration could occur. Values beyond 9 months of age were not obtained in this or other studies with this strain as the lifespan of the obese-T2DM rats began to decline beyond 10 months of age and seldom exceeded 12 months of age, while the lean phenotype was often observed to survive to 12-15 months of age. Data were analyzed via standard statistical procedures including Students t test and ANOVA. The study was approved by the institutional animal care and use committee (IACUC).
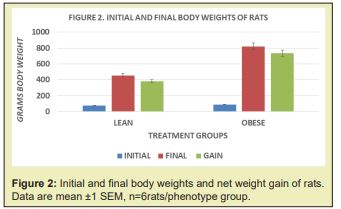
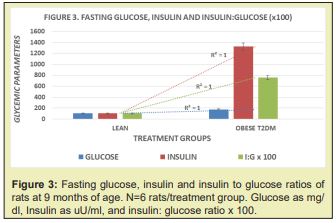
The initial and final body weights of lean and obese animals are depicted in Figure 2, and clearly show that the obese rats while similar in body weight at the onset, gained considerably more weight and were nearly twice as heavy as their lean littermates by 9 months of age. In Figure 1, the lean rats are depicted in the left panel, and the obese littermates in the right panel. The net gain is depicted in the grey bar to the right of each panel and depicts the net gain of each phenotype. The fasting glucose, insulin, and insulin to glucose ratios are depicted in Figure 3 and indicate that fasting glucose and insulin concentrations were well within normal ranges in the lean phenotype but were significantly elevated in the obese phenotype. The differences in the Insulin to glucose ratio were also markedly elevated in the obese-T2DM phenotype, consistent with significant insulin resistance among the obese phenotype of this strain. The trend lines for each parameter gave a linear r2 of 1.0. As noted above, values beyond 9 months of age were not obtained in this or other studies with this strain as the health and lifespan of the obese-T2DM rats was observed to deteriorate prior to 12 months of age.
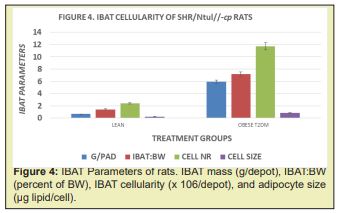
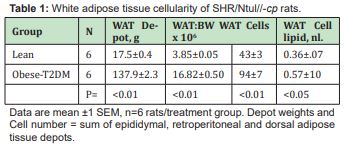
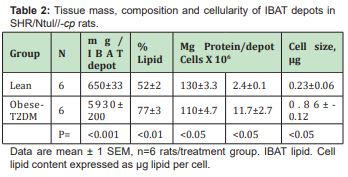
The results of phenotype on cellularity and relative adiposity of multiple white adipose tissue depots are shown in Table 1 and indicate that the mass of combined white adipose tissue depots and the relative adiposity of obese was significantly greater than was observed in the lean phenotype. The hyperplasia and hypertrophy of the WAT is reflective of the greater WAT mass, in that both adipocyte number and adipocyte size averaged an approximate 2-fold increase over their similarly fed and reared lean littermates. The effects of phenotype on the mass and cellularity of the interscapular brown adipose tissue (IBAT) are shown in Table 2 and Figure 4 and indicate that the mass of the IBAT of the obese-T2DM rats greatly exceeded the IAT mass of their lean littermates. Of interest, the usual deep brownish coloration of the IBAT of the obese-T2DM rats was less intense than occurred in their lean littermates, consistent with the greater percent lipid content and lower protein content in those animals. The adipose cellularity of obese-T2DM rats greatly exceeded the cell number per depot in the lean littermates, suggestive at least in part that the hyperphagia common to the obese phenotypes of this and other strains of obese rats may have contributed to the IBAT hyperplasia. IBAT cell size as determined by cell lipid content was also greater in the obese than the lean phenotype and is likely suggestive of diminished endogenous thermogenic activity under thermal neutral conditions. In earlier studies performed early in the course of T2DM development, the resting metabolic rates were observed to be 17% lower in obese than in the lean littermates, while both phenotypes responded to exogenously administered norepinephrine.22
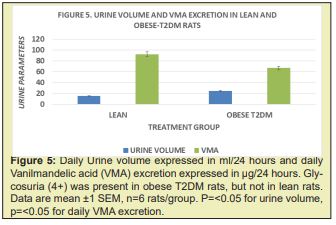
The results of phenotype on urinary volume, glycosuria and daily (24 hour) vanilmandelic acid (VMA) excretion are depicted in Figure 5 and indicate that urine volume was greaterand reflected significant glycosuria in the obese T2DM compared to the lean non-diabetic animals. In contrast, urinary VMA excretion of lean animals was significantly greater than was observed in the obese phenotype, indicative of less endogenous sympathetic (SNS) activity among obese-T2DM animals, despite having a greater mass and cellularity of IBAT but indicative of lesser magnitude of SNS activity.
This study represents the first definitive study on adipose cellularity of the brown adipose tissue in the SHR/Ntul//-cp T2DM rat. BAT is a major contributing tissue of non-shivering thermogenesis, and in lean non-diabetic animals becomes increased in both mass and cellularity in addition to exhibiting greater thermogenic activity during dietary over nutrition, cold exposure and following the feeding of calorically adequate but protein restricted diets otherwise typical of protein energy malnutrition (PEM) dietary regimens.23,24 In the present study, all animals received a nutritionally adequate diet, ad libitum from 4 weeks until 9 months of age, thereby representing much of the typical lifespan of this strain of rat.8,21 The IBAT development occurred normally in the lean phenotype, but the IBAT depots of the obese-T2DM rats were significantly increased by processes of both hyperplasia and hypertrophy in the obese-T2DM phenotype. While increases in BAT mass and cellularity would normally be expected to result in corresponding increases in sympathetic activity, and reflected by corresponding increases in urinary VMA excretion, the greater mass and cellularity of the obese-T2DM phenotype was associated with less sympathetic activity that would have been expected in the absence of obese T2DM stigmata. These results are thus indicative of decreased SNS activity and a decreased endogenous capacity for NST. In previous studies, the obese T2DM phenotype was found to exhibit an impaired capacity to maintain core body temperature when exposed to a 4°C cold environment consistent with the decreased SNS activity resulting in less VMA excretion than occurred in lean animals of the present study.22 The extent to which the decreased SNS activity at 9 months of age may have contributed to the net fat accretion in adipose depots is speculative, but is likely a contributing metabolic factor along with the hyper insulinemia also observed. Insulin exerts numerous hormonally linked biochemical effects in peripheral tissues where it is widely regarded as a lipogenic hormone capable of promoting both de novo lipid biosynthesis in liver and adipose tissue in addition to impaired lipid mobilization in adipose tissues.9,10 The deposition of both dietary and de novo formed lipids are readily deposited into adipose tissue with a minimum of energy expenditure, further enhancing the caloric efficiency of the obese-TDM phenotype. Thus, the mass and cellularity of BAT is not by itself a reliable marker of endogenous thermogenic activity, but may reflect dietary, environmental, genetic and age-related influences in its development and thermogenic activity. In the earlier studies, the RMR of 3 months-old obese-T2DM rats was observed to be significantly lower than was observed in their lean littermates, while exogenous norepinephrine administration at a sub maximal dose of 100 µg/jg BW, subcutaneously resulted in increases in NST of similar magnitude in both phenotypes at that age.22 The onset of T2DM first appears at approximately 10 weeks of age in the obese phenotype of this strain.21,22
The IBAT was found to have a decreased intensity of pigmentation in the obese-T2DM rats, a greater percent lipid content and a proportionally decreased protein content when compared to the IBAT from similarly reared lean littermates. The greater proportion of lipid in the IBAT from the obese-T2DM rats is consistent with the recent reports of the presence of beige adipose tissue in other strains of obese rats by other authors and would be expected as the normally rich brownish coloration of BAT becomes diluted with additional fat accretion.25,26 In BAT, the multiple lipid locules common to brown adipocytes undergo a transition in relative thermogenic activity that includes lipogenic volumetric expansion, while retaining their unique histologic structure of a centrally located nucleus and multiple but now larger lipid locules. The administration of β-blocker agents has also been shown to result in histologic evidence of larger lipid locules in brown adipose tissue.27,28 This observation is consistent with decreased mobilization of lipid from the multiple lipid locules normally present in brown adipocytes, and a decrease in cellular histologic evidence of thermogenic activity of BAT tissues following β-adrenergic blockade.27 In acute starvation, where resting metabolic rates becomes decreased the locularity of brown adipocyte lipid locules has been shown to become decreased in histologic examination as reflected by increased BAT activity likely contributes to thermoregulation in the absence of dietary energy. In contrast, white adipocytes display a single large lipid deposit, surrounded by a thin rim of cytoplasm and a peripherally located flattened nucleus, and a cellular lipid content that often exceeds 1µg of lipid in obese rodents. The combined metabolizable locular surface area of the multiple lipid locules of brown adipocytes far exceeds the metabolizable surface of the single larger lipid droplet commonly present in while adipocytes, thereby enabling a more rapid mobilization of FFA for activation and support of the thermogenic process of brown adipose tissues.
Daily caloric intake is a principal factor is determining numerous parameters of energy metabolism, including hormonal contributors such as insulin and measures of thyroidal activity. In the absence of obesity or the obese-T2DM phenotype, increases in daily caloric intake would be predicted to result in increases in the plasma concentrations of both insulin and thyroid hormones, including T3, with corresponding effects in the metabolism of peripheral tissues. Gavin has shown that the peripheral generation of T3 from T4 is impaired in NIDDM, secondary at least in part to the insulin resistant stat commonly present in NIDDM.29 While measures of thyroidal activity were not quantified in the present study, other studies in obese rats bearing the same -cp trait and similar evidence of insulin resistance and impaired thyroidally-mediated actions including measures of NST were impaired in both aging and obesity.22,30-31
Although daily caloric intake was not determined in the present study, the increases in IBAT mass and cellularity are consistent with the hyperphagia noted elsewhere among obese animals bearing the same epigenetic -cp trait. The characteristics of non-shivering thermogenesis have been reported to be impaired in both obese and obese-T2DM rats bearing the -cp trait, despite having a greater mass of BAT than occurs in their lean littermates.20,32 While measures of metabolic rate or the thermogenic impact of exogenously administered norepinephrine were not included in the current study, norepinephrine is the neurohormone that normally innervates BAT, and the greater IBAT mass exhibited hyperplasia and hypertrophy of brown adipocytes and would be expected to demonstrate an increased capacity for NST independent of the stigmata of obesity and T2DM.1-3,5 In the presence of the obese-2DM stigmata and its associated insulin resistance however the endogenous capacity for NST would likely become impaired, thereby enabling a greater efficiency of caloric utilization.7-10 Additionally, over the duration of this study, an increase in hyper plastic-hypertrophic adiposity and fat accretion was reflected in principle adipose tissue depots. In the present study, the T2DM of obese animals remained untreated, resulting in significant hyper insulinemia, insulin resistance and excess fat accretion in principal and easily quantified depots. The extent to which dietary or pharmacologic control of the obese-T2DM might facilitate a restoration of thermogenic activity and processes of disordered metabolism and caloric efficiency are unknown, and remain an interesting possibility for further study. If such measures were to be applied to affected individuals of industrialized societies one may speculate that proper management could improve the health, wellbeing, and economic burdens of global populations.
The authors thank Dr Carl Hansen of the VRB, NIH for the generous donation of breeding stock, and the late Dr O.E. Michaelis for material contributions to this study.
- 1. Cannon B, Nedergaard J. Brown Adipose Tissue: Function and Physiological Significance. Physiol Rev. 2004;84(1):277–359.
- 2. Himms Hagen J. Role of thermogenesis in brown adipose tissue in regulating energy expensditure. In: The Adipocyte and Obesity: Cellular and Molecular Mechanisms. A Angel (Ed) New York Raven Press, 1983. pp259–270.
- 3. Rothwell NJ, Stock M. A role for brown adipise tissue in diet induced thermogenesis. Nutritin & Metabolism. 1997;5(6):650–656.
- 4. Tulp OL, Gambert S, Horton ES. Adipose tissue development, growth, and food consumption in protein malnourished rats. J Lipid Res. 1979;20(1):47–54.
- 5. Tulp OL, De Bolt SP, Awan AR, et al. The postprandial effects of diet induced thermogeneis in congenic lean and obese LA/Ntul//-cp rats. In: Emerging Trends in disease and health research. 2022;5:93–104.
- 6. Tulp OL, ES Horton. The effect of prolonged experimental protein malnutrition and of refeeding on growth and adipose tissue development in the rat. J Nutr. 1981;111(7):1145–1156.
- 7. Marette A, Tulp OL, Bukowieki LJ. Mechanism linking insulin reistance to defective thermogenesi in brown adipose tisue of obese-diabetic SHR/N-cp rats. Int J Obes. 1991;15:823–831.
- 8. Tulp OL. Charactics of thermognesis, obesity and longevity in the LA/Ntul//-cp rat. ILARJ. 32:32–39
- 9. Maliszewska K, Kwetowski A. Brown adipose tissue and its role in insulin and glucose homeostasis. Int J Mol Sci. 2021;22(4):1530–1536.
- 10. Danforth E Jr. Hormonal conrrol of thermogenesis. Life Sci. 1981;28:1821–1827.
- 11. Tulp OL, Awan AA, Lewis N, et al. Can epigenetic expression contribute to the development of an obese phenotype? Adv Obes Weight Manag Control. 2021;11(3):98‒101.
- 12. World Health Organization: world health organization, 2018.
- 13. Young NL, OL Tulp. “Effect of norepinephrine and nutritional status on metabolic rate in lean and obese LA/N-cp rats”. Comp Biochem Physiol. 1989;94A:597–604.
- 14. Tulp, OL, Awan AR, George P, et al. Treatment with α-methyl paratyrosine inhibits sympathetic but not thyroidal responses to diet-induced thermogenesis in lean Cafeteria-overfed rats. Current Trends in Toxicology and Pharmacology Research. 2022;2(1):1–6.
- 15. Kelly T, Yang CS. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32(9):1431–1437.
- 16. Greenhouse DD. New Models of genetically Obese rats for studies in diabetes, heart disease and complicatios of obesity. ILARJ. 1990;32(3):1–5.
- 17. Hansen CT. Two newcongenic rat strains for nutrition and obesity research. Fed Proc. 1983;42:537.
- 18. Tulp O, Hansen CT, Michaelis OE IV. Non shivering thermogenesis in the SHR/N-cp (Corpulent) rat. Physiol Behav. 1986;36:127–131.
- 19. Hirsch J, Galllian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968;9(1):110–119.
- 20. Tulp OL, Einstein GP. Thermogenesis, aging and obesity in the LA/Ntul//-cp rat. Adv Obes Weight Manag Control. 2021;11(2):37–43.
- 21. Michaelis OE IV. The spontaneously hypertensive/NIH corpulent rat. In: New Models of Genetically Obese Rats for Studies in Diabetes, Heart Disease, and Complications of Obesity. Veterinary Resources Branch, Division of Research Services, NIH publication, Bethesda, MD. 1990;32(3):143-148.
- 22. Tulp OL, Hansen CT, Michaelis ORIV. Nonshivering thermigenesis in the diabetic SHR/N-cp (corpulent) rat. Physiol & Behav. 1986;36:127–131.
- 23. Tulp OL. The development of brown adipose tissue during experimental over-nutrition in rats. Int J Obesity. 1981;5(6):579–591.
- 24. Tulp OL, Gambert S, Horton ES. Adipose tissue development, growth, and food consumption in protein malnourished rats. J Lipid Res. 1979;20(1):47–54.
- 25. Rajan S, Gupta A, Shankar, K. et al. Adipocyte trans differentiation and its molecular targets. Differentiation. 2014;87(5):183–192.
- 26. Rahman MS, Einstein GP, Tulp OL. Autonomic, Immunological and Endocrine influences on adipose tissue as an organ. AOWMC. 2021;11(2):48–58.
- 27. Tulp OL, Root D, R Frink. The effect of anti-hypertensive drug treatment on brown adipocyte diameter and locule distribution in rats. Comp Biochem Physiol. 1984;79C(2):317–320.
- 28. Tulp OL. Effects of acute starvation on brown adipose tissue status in the rat. Nutr Rept Int. 1983;28(2):227–233.
- 29. Gavin LA, McMaHon FA, Moeller M. The mechanism of impaired T3 production from T4 in diabetes. Diabetes. 1981;30:694–699.
- 30. Koletsky S. New type of spontaneouslay hypertensive rat. With hyperlipidemia and endocrien defects. In: Spontaneous hypertension, its pathogenesis and complications. (K.Okamoto, Ed). Tokyo: Igaky Shoin Ltd. 1977. Pp.194-197.
- 31. Tulp OL, CT Hansen, OE Michaelis IV. Effect of dietary carbohydrate and phenotype on thyroid hormones and brown adipose tissue locularity in adult LA/N –cp rats. Comp BiochPhysiol. 1989;94A(2):225–229.
- 32. Tulp OL. Effect of aging and obesity on energy intake, glycemic status and thyroidal actions in the congenic LA/Ntul//-cp rat. AOWMC. 2022.

