Background: Scientific evidence regarding the best dose of corticosteroids for the treatment for Chikungunya virus (CHIKV) infection in the subacute phase to relieve symptoms is lacking.
Objectives: This study aimed to evaluate the effect of corticosteroid therapy on the treatment of subacute Chikungunya.
Methods: This prospective cohort study involved patients with subacute CHIKV treated at the Tropical Medicine Center of Pará Federal University, Brazil. The data were collected between January 2019 and January 2020 during the following two periods: the initial consultation of patients and the return visit. A physical examination, in which the occurrence of inflammatory signs was checked and the number of painful and swollen joints was evaluated, was performed by a rheumatologist.
Results: Data from 65 subjects were analysed with a predominance of females (80%), a mean age of 53.5±13.5 years and a disease duration of 50.0±20.8 days. In this study, 75.4% of the patients used corticosteroids, 73.8% of whom progressed to chronic disease and 26.2% of whom were cured. Although the re-evaluation consultation showed evident improvement in the initial symptoms, the use of a cumulative dose of corticosteroids above 350 mg of prednisone did not affect the outcomes compared to doses less than or equal to 350mg of prednisone.
Conclusion: In conclusion, for patients infected with CHIKV in the subacute phase, doses of prednisone above 350mg do not affect the outcome, and the preference for the use of corticosteroids at the lowest dose for the shortest possible time should be reinforced.
Keywords
Chikungunya virus, Arthralgia, Arthritis, Corticosteroids, Treatment
Chikungunya virus (CHIKV) is a flavivirus belonging to the family Togaviridae and genus Alphavirus. The geographical distribution of CHIKV has been demonstrated most intensely in countries in Africa and Asia, and the migratory flow of travellers facilitates the spread of the virus.1 In Brazil, the first documented case of in digenous transmission occurred in the second half of 2014 in the municipality of Oiapoque, Amapá2 The disease caused by CHIKV is subdivided into acute, subacute and chronic phases, dependingon the time of instillation. During the subacute phase, which is the subject of this article, from 14 days to 3 months of illness, there is a cessation of the febrile condition with a persistence or worsening of pre-existing symptoms.3 The manifestations include arthritis/ arthralgia, oedematous polyarthritis of the fingers and toes, pain, morning stiffness and severe tenosynovitis (especially of the wrists, hands and ankles).4 During the subacute phase, the use of common analgesics (paracetamol or dipyrone)/opioids is recommended; however, non-steroidal anti-inflammatory drugs (NSAIDs) and/or adjuvant medications (anticonvulsants or antidepressants) can be used to treat pain in refractory cases.5 In patients with moderate musculoskeletal pain, the French Guidelines6 recommend using prednisone 10mg/day for five days and withdrawal over 10 days; for severe cases, a regimen of 0.5mg/kg/day for five days and dose reduction within 10 days is used. The Ministry of Health of Brazil and a review conducted by de Brito et al.7 recommend using 0.5mg/ kg/day (maximum dose 40mg/day) until symptom resolution, followed by gradual withdrawal of 5mg/week, not to exceed 21 days of treatment.3,7 Considering the lack of uniformity in the therapeutic proposals at this stage of the disease, especially regarding the dose and time of corticosteroid use, in addition to the understanding that a higher dose and longer use of corticosteroids lead to greater chances of adverse effects, the present study was carried out to evaluate the effect of corticosteroid therapy on the treatment of patients with CHIKV infection during the subacute phase.
A prospective cohort study was carried out involving patients with CHIKV in the subacute phase treated at the Tropical Medicine Center of Pará Federal University (TMC-UFPA), Brazil. The data were collected from January 2019 to January 2020. During this period, 96 patients in the subacute phase were treated; after applying the inclusion and exclusion criteria, a sample of 65 patients was obtained for this research. Patients of both sexes aged 18 years or older with a previous diagnosis of CHIKV infection were included. All patients received a diagnosis of CHIKV based on the positivity of serological tests8,9 and confirmation of the diagnostic criteria for Chikungunya virus fever.10 Patients who had any cognitive impairment that precluded the interview, patients with previous autoimmune musculoskeletal diseases, patients with incomplete forms and patients who refused to participate were excluded.The data were collected during the following two time periods: the initial consultation of the patients and the return visit. In addition to information, such as sex, age and time of symptom onset, data related to the clinical manifestations were collected, and a physical examination, in which the occurrence of inflammatory signs was checked and the number of painful and swollen joints was assessed, was performed by a rheumatologist. The sample was characterized using a database created with Microsoft® Office Excel® 2016 software. To apply descriptive statistics, measures of position, such as the arithmetic mean and standard deviation, were calculated. Analytical statistics were used to evaluate the results of the categorical variables using the G-test and chi-square test of adherence in the univariate analyses and the G-test of independence in the bivariate analyses. In the comparative analyses between the first and second consultations, the chi-square test and G-test were applied. BioEstat ® 5.3 software was used to perform the descriptive and analytical statistical analyses. For decision-making purposes, the 5% level of significance was adopted.
Ethical considerations
This study was conducted in accordance with the principles of the Declaration of Helsinki after receiving approval from the Research Ethics Committee of the TMC-UFPA under registration number 3.001.616 (CAAE 87376518.9.0000.5172) on November 6, 2018.
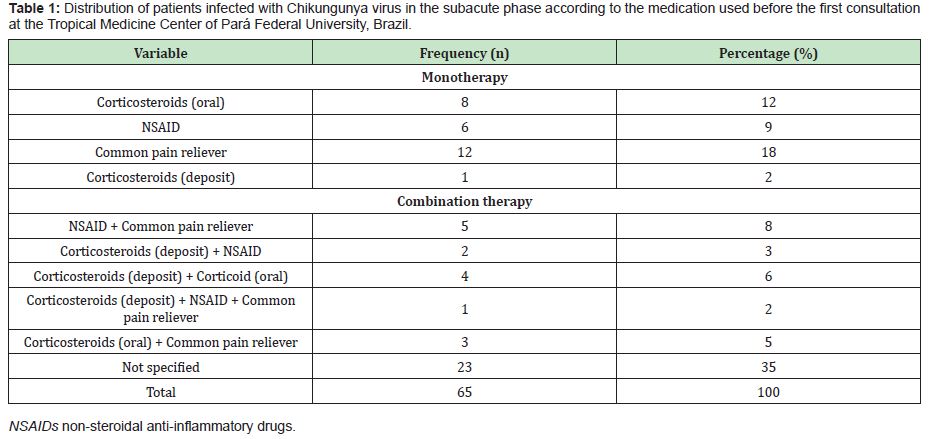
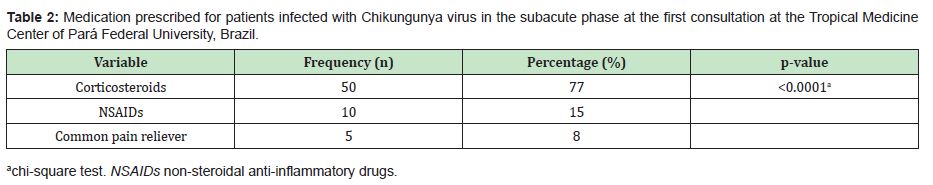
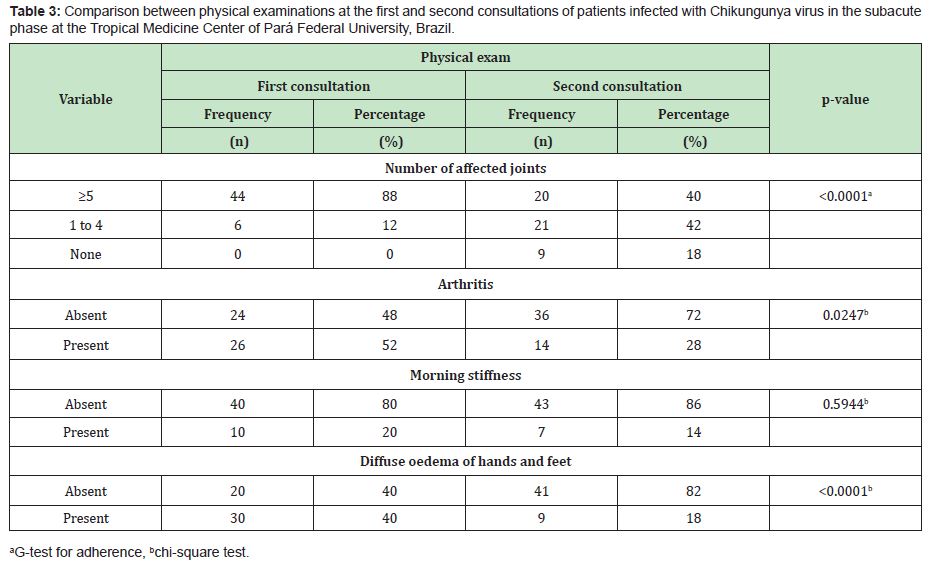
In the present study, 65 patients meeting the criteria for CHIKV infection in the subacute stage of the disease were evaluated. Among these patients, the predominance was female (80%), with a mean age of 53.5±13.5 years (median of 53 years) and disease duration of 50.0±20.8 days (median of 60 days). The most frequent comorbidities were arterial hypertension (27.5%), diabetes mellitus (12.5%) and dyslipidaemia (7.5%). Before attending the TMC-UFPA, it was observed that the patients used drugs to control pain and other initial symptoms of the disease. Among those in which it was possible to identify the treatment performed, the following three classes of drugs were a part of the therapeutic arsenal: common analgesics (paracetamol or dipyrone), NSAID and corticosteroids (oral or injectable deposit formulation). Of these patients, 41.54% used monotherapy, and 23.08% used combined therapy (Table 1). In this study, three classes of drugs were used to treat the patients in the subacute phase of the disease, and corticosteroids were the most frequently prescribed (75.4%) (Table 2). The patients were grouped according to the following two outcomes: cure (those who were completely asymptomatic) (n=17/26.2%) or entry into the chronic phase of the disease (those who maintained signs and/or symptoms for more than 3 months) (n=48/73.8%) (p<0.0001).To establish the effect of corticosteroid use, among the 50 patients who used this medication, their physical examination findings at the first and second visits to the TMC-UFPA were compared. The average time between consultations was 30.5±7.5 days. An improvement in all evaluated parameters was noted (Table 3). NSAIDs and analgesics were used in cycles of 14 days. The standard dose of corticosteroids was 20mg/day of prednisone, with a reduction of 5mg/week; however, changes in the prescription resulted from the peculiarities of each case. In some cases of more intense and disabling symptoms, higher doses were chosen. At other times, the patients themselves increased the dose and/or duration of their medication use. Thus, to facilitate the data analysis, we divided the use of corticosteroids according to the cumulative dose: ≤350mg of prednisone (representing the maximum dose of 20mg with a reduction of 5mg/week) (n=13/26%) and >350mg of prednisone (representing the highest and/or longest dose) (n=37/74%).There was no statistically significant difference in relation to the cumulative dose of this medication between the outcome of the disease and the use of corticosteroids (Table 4).
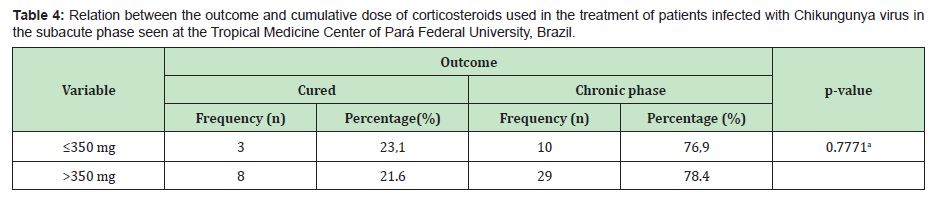
This study revealed that the use of high doses of corticosteroids (cumulative dose>350mg of prednisone) does not improve outcomes related to healing or chronification in individuals with subacute CHIKV infection. This finding is important, as it can prevent patients from being subjected to excessive and/or prolonged doses of corticosteroids, which could increase the occurrence of adverse effects of the medication.The recommended drugs for the treatment of the subacute phase of Chikungunya Fever are analgesics, NSAIDs and corticosteroids.3,5,6 The use of corticosteroids in this condition is a common practice depending on the physician's experience in using this medication. The ability to provide a more objective recommendation, especially for primary care physicians, can facilitate the adoption of safer practices. For example, according to a study by Simon et al.6 in 6 severe cases of musculoskeletal pain, the acceptable dose of prednisone can reach 0.5 mg/kg/ day for 5 days, followed by a dose reduction over 10 days, not to exceed 4 weeks. In a 70kg patient, this cumulative dose could be approximately 525mg (35mg/5 days; 20mg/10 days; 15mg/5 days; 10mg/5 days; and 5mg/5 days). Even in a 60kg individual, the cumulative dose (500mg) could exceed the recommended dose in this research (≤350mg). In another example, following the recommendations of the Ministry of Health of Brazil3 and de Brito et al.7 the dose of prednisone should be 0.5mg/kg/day (maximum dose 40mg/day) until the resolution of symptoms, followed by gradual withdrawal of 5mg/week, not to exceed 21 days of treatment. For example, a 70kg individual could receive 630 mg (35mg/7 days; 30mg/7 days; and 25mg/7 days). Notably, in practice, a dose of 25mg/day of prednisone could not be stopped abruptly after 21 days due to the risk of triggering adrenal insufficiency. The reduction should be gradual, which could further increase the cumulative dose to be used by the patient.
Thus, the dose usually used in the TMC-UFPA outpatient clinic, i.e., 20mg of prednisone/7 days and a reduction of 5mg/week, results in a cumulative dose of 350mg, representing practical and objective guidance, and has shown results similar to those with higher doses.In the present study, it was not possible to carry out a detailed analysis of the use of deposit corticosteroid, as these drugs were prescribed for only 8 patients; however, in cases of more obvious symptomatology, in Brazil, the prescription of corticosteroid injection is common. A medication widely used for this purpose in Brazil includes 7mg of betamethasone, which is equivalent to 70mg of prednisone. In these situations, the prednisone dose that follows the use of the deposit corticosteroid could have the following scheme: 20mg/5 days; 15mg/5 days; 10mg/5 days; and 5mg/5 days. The total cumulative dose obtained by adding the injectable and oral regimen could be 320mg. In practice, an initial dose of injectable corticosteroids actually results in the rapid relief of symptoms in the most serious situations.Based on the results of the Chikbrasil Cohort, the Brazilian Society of Rheumatology suggests the use of lower doses (5 to 20mg/day) of prednisone or prednisolone, with slow and progressive reduction according to the resolution of joint symptoms, as a study demonstrated that the use of corticosteroids in the subacute phase led to a more significant clinical improvement with doses above 10mg/day. However, no additional benefit was obtained from using more than 20mg/day of prednisone.5
In the present study, the patients were re-evaluated after the institution of treatment with corticosteroids. A noticeable reduction in the number of affected joints was observed, resulting in 40% with 5 or more affected joints, which is much lower than the initial 88%. Similarly, in the second assessment, 72% had no signs of arthritis, 86% had no morning stiffness, and 82% no longer had diffuse oedema of the hands and feet.The disease outcome following the treatment employed demonstrated a predominance of chronic evolution (73.85%). These rates are considered high, suggesting that the treatment performed is capable of clinically improving the patients’ condition but not avoiding chronic outcomes in most cases. The data in the literature indicate a chronicity rate of 25% to 75%, while widely varying among studies.3,11–13 This variability may be due to methodological or geographical differences and the presence of comorbidities. It is relevant to highlight a limitation of this study. We were unable to quantify the dose of corticosteroids used prior to the admission of the patients to TMC-UFPA. We chose not to include these data to avoid errors in patient recall, as some reported doses differ from those used, which could influence the analysis.
It is concluded that for patients infected with CHIKV in the subacute phase, doses of prednisone above 350mg do not affect the outcome, and the preference for the use of corticosteroids at the lowest dose for the shortest possible time should be reinforced.
None.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
The authors declare that there is no conflict of interest.
A written informed consent was obtained from all the participants included in this study and no identifying information of any participant was included in this paper.
JAP, VWV, MSMQ, RCMS, CAMC were responsible for data collection and analysis. JAP, VWV, CAMC contributed to the writing of the manuscript. CAMC revised the manuscript.
- 1. Cleton N, Koopmans M, Reimerink J, et al. Come fly with me: review of clinically important arboviruses for global travelers. J Clin Virol. 2012;55(3):191–203.
- 2. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Boletim Epidemiológico. Monitoramento dos casos de dengue até a Semana Epidemiológica (SE) 36 e febre de chikungunya até a SE 37 de 2014. Vol 45. No 21. Setembro de 2014. Brasília: Ministério da Saúde, 2014.
- 3. Ministério da Saúde (BR). Secretaria de Vigilância em Saúde. Chikungunya: manejo clínico. Brasília: Ministério da Saúde, 2017.
- 4. Parola P, Simon F, Oliver M. Tenosynovitis and vascular disorders associated with Chikungunya virus–related rheumatism. Clin Infect Dis. 2007;45(6):801–802.
- 5. Marques CDL, Duarte ALBP, Ranzolin A et al. Recomendações da Sociedade Brasileira de Reumatologia para diagnóstico e tratamento da febre chikungunya. Parte 1 – Diagnóstico e situações especiais. Rev Bras Reumatol. 2017;57(supl.2):s421–s437.
- 6. Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore.) 2007;86(3):123–137.
- 7. De Brito CAA, von Sohsten AKR, Clezio Leitão CCS, et al. Pharmacologic management of pain in patients with Chikungunya: a guideline. Rev Soc Bras Med Trop. 2016;49(6):668–679.
- 8. Galo SS, González K, Téllez Y, et al. Development of in–house serological methods for diagnosis and surveillance of chikungunya. Rev Panam Salud Publica. 2017;41:e56.
- 9. Johnson BW, Russell BJ, Goodman CH. Laboratory diagnosis of Chikungunya Virus infections and commercial sources for diagnostic assays. J Infect Di. 2016;241(Suppl 5):S471–S474.
- 10. Wahid B, Ali A, Rafique S, et al. Global expansion of chikungunya virus: mapping the 64–year history. Int J Infect Dis. 2017;58:69–76.
- 11. Couzigou B, Criquet–Hayot A, Javelle E, et al. Occurrence of Chronic Stage Chikungunya in the General Population of Martinique during the First 2014 Epidemic: A Prospective Epidemiological Study. Am J Trop Med Hyg. 2018;99(1):182–190.
- 12. Schilte C, Staikovsky F, Couderc T, et al. Chikungunya virus–associated long–term arthralgia: a 36–month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137.
- 13. Sissoko D, Malvy D, Ezzedine K et al. Post–epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15–month period. PLoS Negl Trop Dis. 2009;3(3):e389.

