Bacillus Calmette Guerin administered by the aerosol route may locally elicit and increase T1 lymphocyte response in pulmonary alveoli, which are the main target of SARS-2-CoV-2 and its variants, thus preventing and anticipating the virus attack. This may lead to a decrease of the most severe forms of the disease considered as an acute autoimmune virus inducted disease. The lymphocyte response may start to work before and regardless of anti-CoVid vaccination.
Since November 2019 a pandemic infection due to the SARS-CoV-2 virus is still expanding in the world through human-to-human transmission. It affects the respiratory tract, mainly the lungs, but in its most serious forms can also impact other major organs such as kidney, intestine, heart and brain. This infection has the following characteristics:
- It is rapidly contagious via airborne droplets containing viral particles from the mouth and nose through the larynx and the bronchi.
- It has various effects, which may range from infection without clinical manifestations to light or moderate pulmonary symptoms with spontaneous recovery, to rapidly evolving severe forms with serious respiratory insufficiency requiring forced oxygenation, with multi-organ failure and death.1 The characteristics of CoVid-19 pneumonia are different from those of bacterial pneumonia.2,3
- The virus may undergo rapid changes and mutations, which are likely to increase its contagiousness4,5 and consequent lethality.
- Severe forms are more frequent in older patients or patients with pre-existing comorbidities, such as diabetes and arterial hypertension.6
- In severe forms the amount of CD4 and CD8 lymphocytes has been significantly reduced in comparison with lighter forms and this has been attributed to an immunological impairment whose defense might be overcome by the virus.7,8
- In the progression of the disease from "mild" to "severe" forms, a "cytokine storm reaction", "leukocyte changes" and vascular intracoagulation9 occur and the presence of viral particles has been demonstrated in the bronchial mucosa or in the alveoli.10 It has been hypothesized, however, that global BCG immunization may significantly impact the progression of the SARS-CoV-2 pandemic.11
- BCG vaccination may induce a "heterologous" effect on “trained innate immunity”, which may be recalled through non-specific BCG-immunostimulation.12,13 The administration of various kinds of vaccines for the production of IgM and IgG serum antibodies against CoVid-19 has begun. These vaccinations should be given to a large part of the world population as soon as possible, but the process is slow and complex and various trials are underway.14,15 There is no certainty that these vaccines will be effective against some mutated or varied forms of the virus and on the duration of their immunization over time.
We suppose that CoVid-19 is a relevant immune-pathogenesis disease mainly involving the lungs, which can be more or less mild: local intra-alveolar administration of aerosolized BCG, in advance in a population at risk may alert and stimulate in a non-specific way the “trained native immunity” promoting the recruiting of T lymphocytes and directing them in advance towards the alveoli, which are the major targets of the virus. This may reduce the progression to more severe forms of the disease and the spread of the infection.
The action of aerosolized BCG would consist in directing the response of T lymphocytes, especially CD4+ and CD8+, which are crucial in eliciting a specific and adequate immune response and producing a long-term immunological memory,16 preventing the virus’s action in the alveoli, which tends to dodge these defenses.17 This may occur prior to, or concurrently or regardless of the vaccine becoming available, which could be months away and be subject to a certain degree of uncertainty.18
This hypothesis is different as it gives more importance to no or weak lymphocyte response in severe forms with respect of the action of the virus on the alveoli, because lymphopenia prevents CSR and not the contrary. Viral lung injuries may be the result of weak, late or no action by T-lymphocytes whose components are reduced and in which the absolute numbers of T lymphocytes, CD4+ T cells, and CD8+ T cells decreased in nearly all the patients, and were markedly lower in severe cases (294.0, 177.5, and 89.0×106/L, respectively) than moderate cases (640.5, 381.5, and 254.0×106/L, respectively. It was thought that SARS-CoV-2 infection may affect primarily T lymphocytes, particularly CD4+ and CD8+ T cells, resulting in a decrease in numbers as well as IFN-γ production by CD4+ T cells. These potential immunological markers may be of importance because of their correlation with disease severity in COVID-19.19 Viral lung injuries may be caused by weak, late or no action by T-lymphocytes action rather than by the aggression of the virus.20
This absent or delayed action would facilitate the action of the virus whose interaction with the CD147-spike protein21 on the ACE-2 receptor is essential for SARS-CoV-2’s entry into the lung cells.22 Infections of alveolar macrophage by SARS-CoV-2 might be drivers of the "cytokine storm", which might result in damages in pulmonary tissues, heart and lung, and lead to the failure of multiple organs23 with the consequent CID with changes in circulating leukocyte subsets and cytokine secretion, particularly IL-6, IL-1β, IL-10, TNF, GM-CSF, IP-10 (IFN-induced protein 10), IL-17, MCP-3, and IL-1ra. coagulation and vessels impairments, cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection.24
BCG against other virus and pathogens
According to our hypothesis, lung and multi-organ injuries from Covid-19 could be prevented by stimulating lymphocyte response to BCG before virus attack as it has been observed against metapneumovirus in humans25 and against respiratory syncytial virus in mice.26 A non-specific cellular immunity induced by BCG may facilitate, even with SARS-CoV-2 (which has the same respiratory gate and pulmonary target) at the same time, an effective recruitment of Th1 lymphocytes directed against the virus,27 which is capable of inducing a strong proinflammatory cytokine response blocking the production of type I and III IFNs to SARS-CoV-2. It has been seen that treatment of airway cultures with immune molecules type I or type III interferon (IFN) is able to inhibit SARS-CoV-2 infection and represents a potential antiviral treatment for COVID-19 patients.28
It has been demonstrated that BCG, besides having been used for decades against tuberculosis,29,30 has been successfully used as "carrier" to promote a non-specific immune response, effective also against other bacteria, parasites, and viral pathogens, like Bordetella pertussis,31 HIV,32 Plasmodium falciparum33 and capable of enhancing protection against other respiratory viruses.34 Similarly BCG may be used also to promote an immune response against Sars-2-CoV-2, which has the same gate and the same target of other pulmonary viruses.34
Local intralveolar contact of BCG
This recognition and stimulation could be more effective and faster if induced locally by aerosolized BCG as it can happen with pulmonary tuberculosis.35 In the light of the importance of T lymphocytes decrease, particularly CD4+ and CD8+ cells and their correlation with severity of Covid-19,19 aerosol BCG given in strict contact with alveolus may act as a prime boost capable of eliciting a "trained immunity" and a T-lymphocytes increase, which may prevent contagion or infection but also help to decrease the severity of the disease. The second boost may be represented by subsequent alveolar/virus combination, which may find a more effective and prompt response by BCG pre-prepared T lymphocytes. Indeed what may happen in severe forms of the disease is the fact that the virus is not attacked so quickly and effectively to prevent a sequence of the most serious pathological events, mainly pulmonary, of Covid-19 disease.
Covid-19 and autoimmune diseases
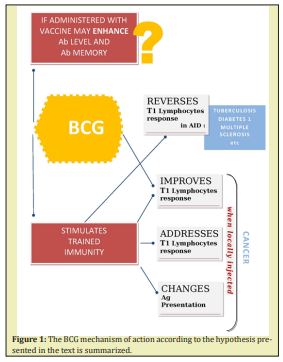
The idea came from the similarities observed in moderate and serious forms of Covid-19 and pulmonary tuberculosis, considered an autoimmune disease on BCG vaccination is effective,36,37 and other autoimmune diseases (AID). An AID occurs when the immune system attacks self-molecules as a result of a breakdown of immunologic tolerance to autoreactive immune cells. Autoimmune diseases include insulin-dependent diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, scleroderma, thyroiditis, and multiple sclerosis.38 Our hypothesis considers Covid-19 as a virus induced acute multi-organ autoimmune disease similar to other autoimmune diseases and pulmonary tuberculosis, where CD4 T cells have a dual role, both are required for host protection as each of these cell types also has potential for mediating tissue damage.39
Other autoimmune diseases are virus-associated because in some instances, immune regulatory mechanisms may falter, culminating in the breakdown of self-tolerance, resulting in immune-mediated attack directed against both viral and self-antigens. During this response, cytotoxic autoantibodies, stimulatory autoantibodies, blocking autoantibodies, or cell-mediated autoimmunity may be observed. It seems then likely that some infectious factors, either viral or bacterial, may change the perception of self recognition of the organ self antigens by the same immune system.40 For a long time, viruses have been shown to modify the clinical picture of several autoimmune diseases, including type 1 diabetes (T1D), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), Sjögren's syndrome (SS), herpetic stromal keratitis (HSK), celiac disease (CD), and multiple sclerosis (MS). Best examples of viral infections that have been proposed to modulate the induction and development of autoimmune diseases are enteric virus infections such as coxsackie B virus (CVB) and rotavirus, as well as influenza A viruses (IAV), and herpesviruses. Other viruses that have been studied in this context include measles, mumps and rubella.41 On the other hand, the appearance of autoinflammatory/autoimmune phenomena in patients with COVID-19 calls attention for the development of new strategies for the management of life-threatening conditions in critically ill patients. Antiphospholipid syndrome, autoimmune cytopenia, Guillain-Barré syndrome and Kawasaki disease have each been reported in patients with COVID-19.42 The knowledge about SARS-CoV-2 disease pathogenesis and immune response is a cornerstone to develop rationale-based clinical therapeutic strategies. In addition to that, several studies have recently suggested that immune dysregulation and hyperinflammatory response induced by SARS-CoV-2 are more involved in disease severity than the virus itself.43 As a result, the use of autoimmune disease drugs can be useful in treating COVID-19.44
BCG can prime an immune response in AID and cancer
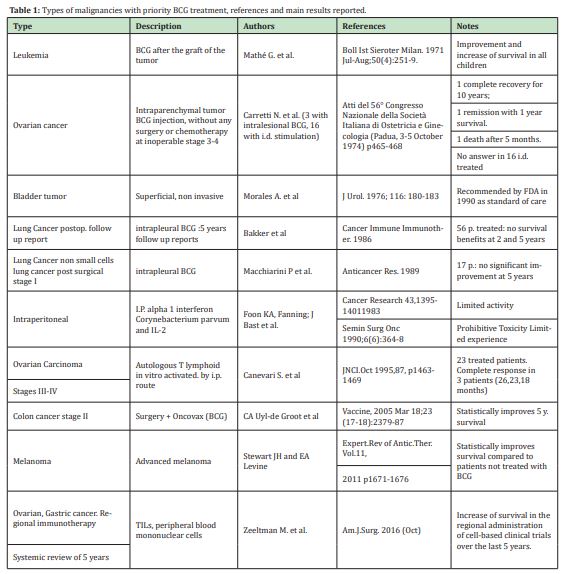
The idea evolved considering analogies between reverse immune reaction with BCG in cancer and AID by stimulating trained immunity. The innate immune system can mount resistance to reinfection, a phenomenon termed trained immunity or innate immune memory. During trained immunity, innate immune cells display gene- or locus-specific changes in their chromatin profiles induced by a previous stimulation. These changes, however, allow increased response to re-stimulation of the cells through both the same and different PRRs.45,46 A large number of murine and human studies taken together suggest that innate immune responses have the capacity to be “primed” or “trained,” by a live vaccine like BCG and to mount a more robust response to secondary non-related stimuli after being initially primed (or trained) by a challenge of BCG.47 Such reprogramming causes a changed immune response to secondary stimuli. These include conditions characterized by excessive trained immunity, such as inflammatory and autoimmune disorders, allergies and cardiovascular disease and conditions driven by defective trained immunity, such as cancer.48 Thereby in order to exert a new type of immunological memory upon re-infection, the non-specific protective effect against Covid-19 offered by BCG through induced trained immunity has been recently discussed.49,50 In Table 1 the types of cancers treated with intralesional BCG are summarized according to their priorities.
- Our hypothesis is that local stimulation of the alveoli with BCG aerosol, as for TB (35) may trigger an early reaction by T1 lymphocytes against the same target of the virus, increasing the non-specific pulmonary defense before the viral attack. The alveolus would represent, in fact, the same target of the BCG, of the virus and of the response of CD4 and CD8 T1 lymphocytes. Pneumonia from COVID might be related to immune system dysregulation with possible production of autoantibody and hyper-triggered response rather than viral infection consequence (3).
- The decrease of blood lymphocytes is higher in severe forms and lower in less severe forms. Lymphocytes in severe forms are alerted too late and will not act in time (16-19-20). The virus may have blinded, bypassed or anticipated the immune response because it is unknown and acts quickly in the alveoli, which are the targets of the disease.17
- BCG may act in preventing not only tuberculosis (29) but also, in an unspecific manner, other bacterial and viral diseases (31-32-33-34) and since an inverse correlation between vaccinations with BCG and COVID has been observed,52 its use has been suggested.49-50
- Local administration of BCG in some human tumors (cancer of the ovary, bladder, melanoma)52-56 results in complete remission of the tumor probably altering antigen presentation and "reversing" the lymphocyte reaction against the new tumor/BCG antigen combination, no longer recognized as "self" by the "trained immunity" that first did not attack it. The use of BCG with intradermal injection of extracts of the same human tumors in patients presensitized only with intradermal BCG was effective only on 2/14 patients.57
The importance of the hypothesis is to decrease or cancel the evolution towards the most serious pulmonary forms of Covid-19 by increasing T1 lymphocytes response through local administration of aerosolized BCG given in advance to patients at risk of infection. We believe that changing antigen presentation of pulmonary alveoli locally with administration of BCG aerosol could stimulate and direct trained immunity against the ensuing action of SARS-CoV-2 on the organ targeted by the disease, as observed in human tumors57,58 by intraparenchymal use of BCG.52 This procedure may be taken into account with regard to elderly patients, patients with heart diseases, diabetes, cancer, immunodepression and all other groups of people at higher risk.
Our hypothesis may have, at least, the following potential implications:
- It could reduce the progression from mild to serious forms of COVID-19 correlated with the detection of a low amount of lymphocytes (1-7) whose defense may be "overcome" by the virus (8) increasing the presence of T lymphocytes (12) and interferon (9-28).
- It highlights the importance of stimulating a response of the native trained immunity using BCG (12-46-47-48) against COVID-19 (49-50) delivered locally by aerosol (35) and directed against the alveoli of the lungs, primary target of the virus infection.10
- This response is faster than a vaccine, which requires more than 30 days to develop the presence of IgG antivirus antibodies, and prevents the virus from dodging guest’s defenses.17
- In fact, the the duration of immunity of current vaccines is not well known.
- It does not depend on genetic variants of the virus4,59,60 and on changes of the CD-147-spike protein which may increase contagion, infectivity and lethality.5
- BCG (formed by inactivated tubercle bacilli) may be administered along with the vaccines, acting as an adjuvant (as happens with Freund’s complete adjuvant) to increase the level of specific serum antibodies, the duration of their immunization over time (58) and, perhaps, having a favourable effect against some virus variants.
- The response to BCG could be tested in advance and, if reduced–as in elderly patients,38-62 cancer patients,63 cardiac patients (heart diseases are present in 8-12% of CoVid cases),64 patients with vascular and atherosclerotic diseases,65 diabetic patients, who are the ones with the worst prognosis in case of severe forms of CoVid-19,66 and others–, can be solicited.57
- The administration is simpler as it can be delivered via aerosol. This route of immunization offers not only the scientific advantage of delivering the vaccine directly to the respiratory mucosa, but also practical and logistical advantages. Being less expensive, it does not require complex structures and medical and nursing organization and it is easier to implement.35
- The result may be subsequently monitored and tested through dermal tests or tests that determine lymphocyte response such as CD4/CD8 ratio: in fact, a renewed interest has emerged about the usefulness of CD4/CD8 ratio as a strong marker of immune activation and immune senescence.67
It anticipates the virus action on AM of Sars-2-Covid characterized by fast contagion, progression and worsening of conditions, which require equally quick responses from the body’s immune system.
Figure 1 we propose the use of local intralveolar BCG given by aerosol route in at-risk Covid-19 subjects with the aim of soliciting, preventing and addressing a “trained immunity” response to possibly neoformed virus-MA Antingen.
The first idea and attempt to use BCG intralesionally first used in a solid human tumor in 1973 may be considered still relevant,52 also in the light of successive developments of cancer immunotherapy with immune checkpoint blockade therapy.68 It is one of the most successful intralesional biotherapies for cancer in use and has been employed in ovarian cancer,52-54 especially to treat non-muscle-invasive bladder cancer in which it has represented the "golden therapy" for more than 30 years,55 and in metastatic melanoma therapy.56 The mechanism of its therapeutic effect is still under investigation. Available evidence suggests that tumor cells (including ovarian cancer or bladder cancer cells themselves) and cells of the immune system both have crucial roles in the therapeutic antitumor effect of BCG. The possible involvement in bladder cancer cells as in other tumors includes attachment and internalization of BCG, secretion of cytokines and chemokines, and presentation of BCG and/or cancer cell antigens to cells of the immune system. It is thought that immune system cell subsets that have potential roles in BCG therapy include CD4(+) and CD8(+) lymphocytes, natural killer cells, granulocytes, macrophages, and dendritic cells.55 However what seems crucial in all the circumstances in which this kind of immunotherapy approach of cancers has been used, is the strict interaction that is created between BCG and tumor cell.52,55
As far as COVID-19 is concerned, our hypothesis considers that BCG given by aerosol route, might work, similarly to what happens in tumors, by altering the antigen presentation of alveoli with which it comes into contact: such as a newly formed combination represented by alveolar/BCG complex may change the immune response of CD4/CD8 T1 lymphocytes which, before this challenge, recognized the alveolar target as "self". This approach may anticipate and prevent the immune reaction of the secondary contact with the virus, which has the same target of locally administered intra-alveolar BCG. This could determine an effective and robust local response by T1 lymphocytes, as it occurs with BCG aerosol in pulmonary TB35 and in tumors (ovary, melanoma, bladder) on which BCG is effective only when directly injected52-54 or in close contact with tumor cells,55 plausibly changing the tumor antigen presentation. It has been seen, in fact, that the down regulation/loss of antigen presentation in cancer is a major immune escape mechanism. It allows tumor cells to become ‘invisible’ and avoid immune attack by antitumor T cells. On the contrary down regulation/loss of antigen presentation may also prevent direct priming of naïve T cells by tumor cells as it happens by forcing tumor cells to present their antigens turning tumor presenting antigen and immune response.69 With regard to protection against COVID-19, our hypothesis is that the contact of BCG on the bronchial and/or alveolar mucosa (first challenge) determines a locally induced change of the "trained immunity” and of the lymphocyte component also against the virus/alveolar macrophages (AM) antigen23 combination after coming into contact with COVID-19 (second challenge). The approach of using an adjuvant system through intranasal route to improve protective effectiveness and elicit a long-term robust immune response has recently proven effective against avian coronavirus.70
As intraparenchymal BCG is effective in treating tumors only if injected locally, in the same way we propose to solicit locally, by intralveolar administration, the trained immunity for the purpose of obtaining a higher T-lymphocyte response after a second challenge on the same target represented by the possible presence of SARS-COV-2. This response would anticipate the virus’s harmful action and progression to more severe forms of the disease. BCG may work locally priming the AM (first boost), changing antigen presentation, stimulating the “trained immunity” in a new manner and directing against this newly formed alveolar BCG modified antigen the T lymphocytes reaction if contact with Sars-2-CoV-2 occurs on the same alveolar target (second boost). The mechanism would be similar to the reaction that occurs in cancer after intralesional injection of BCG: in both cases, cancer and the pulmonary alveoli might be antigenically modified locally by BCG and, subsequently, the T lymphocyte reaction is addressed locally. Thus the use of BCG aerosol may be proposed in order to locally elicit and improve an immune-mediated CD4+ and CD8+ T-cell as well as IFN-γ production by CD4+ T lymphocytes therefore preventing the most severe forms of pulmonary damages due to COVID-19, with the idea of preventing and anticipating the virus’s aggression to the lung's AM which represents its most harmful aspect for the entire sequence of the ensuing events described (CSR and CID). As a conclusion, in our opinion, the Corona virus pandemic should be addressed from an opposite perspective before resorting to vaccination, that is to strengthen in a certain population or higher risk areas (care homes for the elderly, presence of underlying diseases such as hypertension, diabetes, heart diseases chemotherapy treatments, etc) the "trained immunity" in the target organ of COVID-19, the lung, in a non-specific but "matching" manner with local administration of BCG. The strengthening and implementing of T lymphocytes’ action, both locally and a specifically, directing it in advance for the protection of the real target of the disease, the lung, could also reduce the need to chase, with vaccination alone, the continuous changes of the spike protein and of the viral genoma, which can occur very rapidly.
None.
None.
Authors declare that there is no conflict of interest.
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- 2. Perrella A, Trama U, Bernardi FF, et al. Editorial - COVID-19, more than a viral pneumonia. Eur Rev Med Pharmacol Sci. 2020;24(9):5183–5185.
- 3. Ahmet Kursat Azkur, Mübeccel Akdis, Dilek Azkur, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581.
- 4. Salvatore Dimonte,, Muhammed Babakir-Mina, Taib Hama- Soor, et al. Genetic Variation and Evolution of the 2019 Novel Coronavirus. 2021;24(1-2):54–66.
- 5. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827.
- 6. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887.
- 7. Feng Wang, Hongyan Hou, Ying Luo, et al. The Laboratory Tests and Host Immunity of COVID-19 Patients With Different Severity of Illness. JCI Insight. 2020;5(10):137799.
- 8. Guang Chen, Wu D, Guo W, et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest. 2020;130(5):2560–2629.
- 9. Jin Wang, Mengmeng Jiang, Xin Chen, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and the rapy concepts. J Leuc Biol. 2020;108(1):b17–41.
- 10. Yao XH, Li TY, Che Z, et al. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417.
- 11. Mayda Gursel, Ishan Gursel. Is global BCG vaccination-induced trained immunity relevant to the progression of sars-cov-2 pandemic? Allergy (letter to the Editor). 2020;75(7):1815–1819.
- 12. Gyssens IC, Netea MG. Eterologous effects of vaccination and trained immunity. Clin Microbiol Infect. 2019;25(12):1457–1458.
- 13. Binte Aziza Jennifer, L Dembinskib Yasmin Jahan, Debate on Bacille Calmette-Guérin vaccination against COVID-19: Is it worth performing clinical trials?. Biosafety and Health. 2020;2(3):113–114.
- 14. Koirala A, Joo YJ, Khatami A, et al. Vaccines for COVID-19: The current state of play. Paediatr Respir Rev. 2020;35:43–49.
- 15. Jawad Al-Kassmy, Jannie Pedersen, Gary Kobinger. Review Vaccine Candidates against Coronavirus Infections .Where Does COVID–19 Stand?. Viruses. 2020;12(8):861.
- 16. Alba Grifoni, Daniela Weiskopf, Sydney I. Ramirez et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181(7):1489–1501.
- 17. Atul Udgata, Komal Dolasia, Sudip Ghosh et al. Dribbling through the host defence: targeting the TLRs by pathogens. Critical Reviews in Microbiology. 2019;45(3).
- 18. Jawad Al-Kassmy, Jannie Pedersen, Gary Kobinger. Review Vaccine Candidates against Coronavirus Infections. Where Does COVID - 19 Stand?. Viruses.2020;12(8):861.
- 19. Chen G, Wu D, Guo W, et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J Clin Invest. 2020;130(5):2560–2629.
- 20. Azkur AK, Akdis M, Akdis CA, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581.
- 21. Ke Wang, Wei Chen, Yu Sen Zhou, et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein.
- 22. Haoyi Zheng, Jane Cao J. Angiotens in-Converting Enzyme Gene Polymorphism and Severe Lung Injury in Patients with Coronavirus Disease. Am J Pathol. 2020;190(10):2013–2017.
- 23. Infection of alveolar macrophage by SARS-CoV-2 might be drivers of the “cytokine storm”, which might result in damages in pulmonary tissues, heart and lung, and lead to the failure of multiple organs. Chaofu Wang, Jing Xie, Lei Zhao, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. E Bio Medicine. 2020;57.
- 24. Jin Wang, Mengmeng Jiang, Xin Che, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41.
- 25. Christian E Palavecino, Pablo F Cespedes, Roberto S Gomez, et al. Immunization with a Recombinant Bacillus Calmette–Guerin Strain Confers Protective Th1 Immunity against the Human Metapneumovirus. J Immunol. 2014;192:214–223.
- 26. Bueno SM, González PA, Cautivo KM, et al. 2008. Protective T cell immunity against respiratory syncytialvirus is efficiently induced by recombinant BCG. Proc Natl Acad Sci USA. 105:20822–20827.
- 27. Cautivo KM, Bueno SM, Cortes CM, et al. Effcient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guérin promotes virus clearance and protects from infection. J Immunol. 2010;185:7633–7645.
- 28. Abigail Vanderheiden, Philipp Ralfs, Tatiana Chirkova, et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J Virol. 2020;94(19).
- 29. Chika N. Okafor, Ayesan Rewane, Ifeanyi I Momodu, et al. Bacillus Calmette Guerin. [Updated 2020 Jul 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020.
- 30. Baumann SA. Nasser Eddine, SH Kaufmann. 2006. Progress in tuberculosis vaccine development. Curr Opin Immunol. 2006;18(4):438–448.
- 31. Nascimento IP, Dias WO, Quintilio W, et al, 2008. Neonatal immunization with a single dose of recombinant BCG expressing subunit S1 from pertussis toxin induces complete protection against Bordetella pertussis intracerebral challenge. Microbes Infect. 2008;1092:198–202.
- 32. Chapman R, Chege G, Shephard E, et al. 2010. Recombinant Mycobacterium bovis BCG as an HIV vaccine vector. Curr HIV Res. 2010;8(4):282–298.
- 33. Teo WH, Nurul AA, Norazmi MN. Immunogenicity of recombinant BCG-based vaccine expressing the 22 kDa of serine repeat antigen (SE22) of Plasmodium falciparum. Trop Biomed. 2012;29:239–253.
- 34. Zhang ZJ. Surveillance and control of ARI among urban nurseries in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 1990;11:145–149.
- 35. Zita Rose Manjaly Thomas, Helen McShane. Aerosol immunisation for TB: matching route of vaccination to route of infection. Trans R Soc Trop Med Hyg. 2015;109(3):175–181.
- 36. Paul Elkington. Tuberculosis: an Infection-Initiated Autoimmune Disease?. Trends Immunol. 2016; 37(12):815–818.
- 37. Katrin D Mayer Barber, Daniel L Barber. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Springs Harb Prospect Med. 2015;17(5):a018424.
- 38. Smith DA, Germolec DR. Introduction to immunology and autoimmunity. Environ Health Perspect. 1999;107(Suppl 5):661–665.
- 39. Münz C, Lünemann JD, Getts MT. Antiviral immune responses: triggers of or triggered by autoimmunity?. Nat Rev Immunol. 2009;9(4):246–258.
- 40. Daniel R Getts, Emily ML Chastain, Rachael L Terry, et al. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255(1):197–209.
- 41. Maria K Smatti, Farhan S Cyprian, Gheyath K Nasrallah, et al. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses. 2019;11(8):762.
- 42. Yhojan Rodríguez, Lucia Novelli, Manuel Rojas, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506.
- 43. Bruno Bordallo, Mozart Bellas, Arthur Fernandes Cortez, et al. Severe COVID-19: what have we learned with the immunopathogenesis?. Adv Rheumatol. 2020;60(1):50.
- 44. Sahar Najafi, Elham Rajaei, Rezvan Moallemian, et al. The potential similarities of COVID-19 and autoimmune disease pathogenesis and therapeutic options: new insights approach. Clin Rheumatol. 2020;39(11): 3223–3235.
- 45. Kumar H, Kawai T, Akira S. “Pathogen recognition by the innate immune system”. International Reviews of Immunology. 30(1):16–34.
- 46. Benn CS, Netea MG, Selin LK, et al. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34(9):431–439.
- 47. Mihai G Netea, Leo AB Joosten, Eicke Latz, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098.
- 48. Willem JM Mulder, Jordi Ochando, Leo AB Joosten, et al. Therapeutic targeting of trained immunity. Nat Rev Drug Discov. 2019;18(7):553–566.
- 49. Luke AJ O’Neil, Mihai G Netea. BCG-induced trained immunity: can it offer protection against COVID-19?. Nat Rev Immunol. 2020 Jun;20(6):335–337.
- 50. Galina Zhelezova, Valeria Mateeva, Grisha Mateev. Is the BCG vaccine a useful tool against COVID-19?. Clin Dermatol. 2020.
- 51. Willis X Li. Worldwide inverse correlation between Bacille Calmette-Guérin (BCG) immunization and COVID-19 mortality. Infection. 2021;1–11.
- 52. Carretti N. Bacillus Calmette-Guérin (BCG) in immunotherapy of ovarian tumors: from the beginning to recent findings. Is it still up to date?; Italian JOG. 2021;33(1):11–25.
- 53. Carretti N. Treatment with intralesional BCG of subcutaneous metastasis from ovarian tumor and temporal regression of the tumor. Atti della Società Italiana di Ostetricia e Ginecologia. 1974;56:476–479.
- 54. N Carretti, PA Galli, D De Salvia, et al. Considerations about two cases of metastatic and inoperable ovarian tumors treated with BCG inoculation in the neoplastic mass, Atti della Società Italiana di Ostetricia e Ginecologia. 1974;56:465–468.
- 55. Redelman Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer - a current perspective. Nat Rev Urol. 2014;11(3):153–162.
- 56. Benitez MLR, Bender CB, Oliveira TL, et al. Mycobacterium bovis BCG in metastatic melanoma therapy. Appl Microbiol Biotechnol. 2019;103(19):7903–7916.
- 57. Carretti N, PA Galli, D. Minucci. Delayed hypersensitivity “in vivo” by BCG and tumoral extracts in patients with gynecological tumors. Atti della Società Italiana di Ostetricia e Ginecologia. 1974;56:469–475.
- 58. Van Puffelen JH, Keating ST, Oosterwijk E, et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat Rev Urol. 2020;17(9):513–525.
- 59. Zijie Shen, Xan Yiao, Lu Kang et al. Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020;71(15):713–720.
- 60. Sarita Choudhary, Karli Sreenivasulu , Prasenjit Mitra et al. Role of Genetic Variants and Gene Expression in the Susceptibility and Severity of COVID-19. Ann Lab Med. 2021;41(2):129–138.
- 61. Nicola Carretti, Zoltan Ovary. Transmission of gammaG antibodies from Maternal to Fetal Circulation in the Mouse. Proc Soc Exptl Biol Med. 1969;130:509–512.
- 62. Evan J Anderson, Nadine G Rouphael, Alicia T Widge, et al. mRNA-1273 Study Group , Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438.
- 63. Hyun Jee Han, Chinekwu Nwagwu, Obumneme Anyim, et al. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol. 2021;90:107247.
- 64. Manish Bansal. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250.
- 65. Paul C Evans, Ed Rainger G, Justin C Mason, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116(14):2177–2184.
- 66. Lorenzo Lughetti, Viola Trevisani, Umberto Cattini, et al. COVID-19 and Type 1 Diabetes: Concerns and Challenges. Acta Biomed. 2020;91(3):e2020033.
- 67. Bruno G, Saracino A, Monno L, et al. The Revival of an "Old" Marker: CD4/CD8 Ratio. AIDS Rev. 2017;19(2):81–88.
- 68. Spencer C Wei, Colm R Duffy, James P Allison. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069-1086.
- 69. Marie de Charette, Aurélien Marabelle, Roch Houot. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy. Eur J Cancer. 2016;68:134–147.
- 70. Shaswath S Chandrasekar, Brock Kingstad-Bakke, Chia-Wei Wu, et al. A Novel Mucosal Adjuvant System for Immunization against Avian Coronavirus Causing Infectious Bronchitis. J Virol. 2020;94(19):e01016–e01020.

