Background: The objective of the present study was to determine the maximum nonlethal dose of epinephrine, lidocaine, and both substances diluted in saline subcutaneously injected in rabbits.
Methods: The animals were divided into three groups. The first group received a maximum dose of subcutaneous epinephrine diluted in 30mL of saline with decreasing doses of epinephrine: 10.01mg/kg, 7.25mg/kg, 5.32mg/kg, 2.51mg/kg, 1.85mg/kg, 2.8mg/kg, and 1.21mg/kg. The second group received 30mL of saline with decreasing doses of lidocaine 250, 200, 150, 125, 100, 75, and 50mg/kg, respectively. The animals were observed from 48 hours to 7 days after receiving the injection. The third group also received 30mL of saline containing a fixed dose of 0.03mg of epinephrine and the same decreasing doses of lidocaine as the second group. After receiving the injection, the animals were observed for 48 hours for adverse reactions, death, and absence of vital signs.
Results: In the first group, the dose of 1.21mg/kg of epinephrine has proved to be nonlethal. In the second group, a dose of 50mg/kg of lidocaine subcutaneously injected to rabbits caused neither visible reactions nor death. Finally, in the third group, lidocaine plus epinephrine at a dose of 75mg/kg plus 0.03mg in the saline did not cause visible reactions or death.
Conclusion: Lidocaine injected into the subcutaneous tissue of rabbits at a dose of 50mg/kg did not cause visible reactions or death.
Keywords: Lidocaine, Epinephrine, Liposuction, Rabbits
Liposuction is one of the most frequent surgical procedures in plastic surgery. The techniques of body fat removal and remodeling have evolved since the emergence of liposuction.1 The use of anesthesia with abundant infiltration containing large amounts of saline, epinephrine, lidocaine, and bicarbonate.2-5 has made it possible to control blood loss and increase the amount of adipose tissue removed.
The maximum dose of lidocaine recommended in the literature is 7mg/kg of body weight. This dose applies only to perivascular blocks, and it cannot be used in subcutaneous injection for liposuction.6,7 Much larger amounts of lidocaine have been used in subcutaneous injections for liposuction, such as 75mg/kg.8 55mg/kg,4,9 and 35mg/kg10 of body weight, without related side effects.3,9-11 Few studies have been conducted in animals to determine a safe dose of lidocaine for injection in liposuction procedures.3,9-11
Lidocaine is a local anesthetic that was synthesized in 1943 by Lofgren.6 It is derived from cocaine, and it was the first drug to be used as a local anesthetic. The synthesis of lidocaine provided a substance with low local irritation and systemic toxicity, fast action, and long duration of effect. Lidocaine is currently the most popular local anesthetic. Its toxic effects reach mainly the central nervous system (CNS) and the cardiovascular system. At very high doses, lidocaine may cause drowsiness, light- headedness, hearing and visual disturbances, and restlessness. At even higher levels, lidocaine may cause nystagmus, shaking, and tonic-clonic seizures followed by CNS depression and death.6 In the cardiovascular system, local anesthetics depress excitability and conductivity, thus resulting in change of rhythm, decreased strength of cardiac contraction, arteriolar dilation, leading to hypotension with consequent circulatory collapse and death.3
Lidocaine is a fat-soluble substance; therefore, it reacts when in contact with fatty tissues. The adipose tissue retains the local anesthetic, thus delaying its systemic absorption. Patients with a higher percentage of fatty tissue probably retain drugs for a longer time.10 Adrenaline, or epinephrine, is a natural catecholamine. That is, a sympathomimetic amine whose basic substance is beta-phenylethylamine, which promotes the formation of products with sympathomimetic activity. In the human body, there is natural endogenous synthesis of three catecholamines: dopamine, norepinephrine, and epinephrine. Epinephrine is produced by the adrenal medulla from noradrenaline, especially under high stress conditions. The most important action of epinephrine is related to vascular muscle stimulation, inhibition of intestinal or bronchial muscles, stimulation of heart contraction and heart rate, as well as effects on the CNS. Because of the pharmacological properties of epinephrine related to the cardiovascular system, smooth muscles, and CNS, its use in medical practice is essential. However, its use is also controversial because of the systemic repercussions caused by its properties. Epinephrine has been used in outpatient surgeries and minor surgeries because its strong vasoconstrictive action reduces bleeding during surgical procedures when injected into tissues. When epinephrine is used in combination with local anesthetics, it delays the absorption of these drugs. Therefore, using epinephrine as a vasoconstrictor agent in the local anesthetic solution slows its absorption rate.3,12,13 All aspects of the changes caused by epinephrine in the human body have been widely published in the literature.1,14-16 Countless studies on the dose of epinephrine have considered only the intravenous administration. Because of its frequent use in surgery, particularly in plastic surgery, after the introduction of liposuction by Illouz in 1977 for the treatment of lipodystrophy and body contouring and because of the increase in the number of this type of surgery, there has been also a need to increase the dose of epinephrine in combination with the anesthetic substance for subcutaneous administration. Nevertheless, there is no consensus in the current literature on the safe dose of subcutaneous epinephrine. There are only reports on increasing doses of epinephrine administered to patients undergoing liposuction.4,8-10
There is faster absorption of local anesthetics added to concentrated solutions in comparison with diluted solutions containing the same dose, even in subcutaneous administration. The addition of epinephrine results in vasoconstriction and slows down the systemic absorption.10 Therefore; we decided to conduct an experimental study to establish objective criteria regarding epinephrine's action.
The objective of the present study was to evaluate the safe dose of lidocaine, epinephrine, and both substances subcutaneously injected in rabbits.
This study was conducted at the Laboratory of Experimental Surgery of the School of Medicine of our institution. We used Norfolk rabbits of both sexes whose mean weight was 2kg. The animals were divided into three groups: lidocaine group, epinephrine group, and lidocaine plus epinephrine group. The lidocaine group was divided into six subgroups including five rabbits each.
The epinephrine group was divided into six subgroups including 10 rabbits each. Finally, the lidocaine plus epinephrine group was divided into six subgroups including five rabbits each. The injections were applied subcutaneously in the abdominal region of the animals.
The first group received 2% lidocaine at concentrations of 200mg/kg, 150mg/kg, 125mg/kg, 100mg/kg, 75mg/kg, and 50mg/kg, supplemented with saline in a total volume of 30mL. The second group received epinephrine at concentrations of 10.01mg/kg, 7.25mg/kg, 5.32mg/kg, 2.51mg/kg, 1.85mg/kg, 2.8mg/kg, and 1.21mg/kg. Epinephrine was diluted in 30mL of saline.
The animals were observed for up to 7 days after the injections. Clinical signs and deaths were recorded. The third group received 0.03mg of epinephrine added to 30mL of saline containing lidocaine at the same concentrations described for the first group. The animals were placed in appropriate cages and observed from 48 hours to 7 days.
Data regarding the presence or absence of signs such as drowsiness, lethargy, restlessness, and seizures, as well as death were analyzed in each experimental group using the method of generalized linear models17 for randomized trials, considering binomial distribution and canonical link function. The analyses were implemented using the GENMOD procedure of the SAS software (SAS, 2000). The findings were obtained considering a 5% significance level .17,18
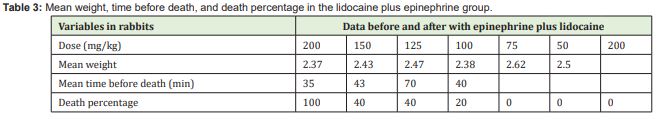
Table 1 presents the results for the lidocaine group. In this group, all rabbits receiving 200mg/kg of body weight had drowsiness, lethargy, restlessness, tonic-clonic seizures, difficulty breathing, nostril flaring, and death. The subgroup that received 150mg/kg showed the same signs and death. The subgroup that received 125mg/kg had the same signs, whereas four animals died and only one survived. The subgroup that received 100mg/kg showed the same signs, but none of them died. In the subgroup that received 75mg/kg, only one rabbit had the same signs and did not die. The other four rabbits showed no changes. In the subgroup of 50mg/kg, none of the rabbits showed any changes.
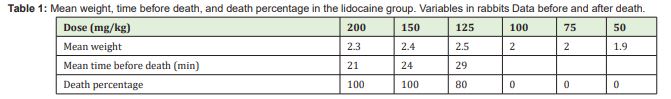
As Table 2 shows, in the group that received only epinephrine, all animals of subgroups of 10.01 of body weight died within 3-24 hours. Immediately after the injection, the rabbits had tachycardia, lethargy, and tachypnea. Later, when they were close to death, the rabbits had epistaxis. These signs were observed in all rabbits except for those receiving a dose of 1.21mg/kg. Among the animals receiving 5.32mg/kg, four died before 24 hours. Among the rabbits receiving 2.51mg/kg, four died before 48 hours. There were no deaths in the subgroup that received 1.21mg/kg. In addition, the rabbits in this subgroup had none of the signs observed in all the animals of the other subgroups.
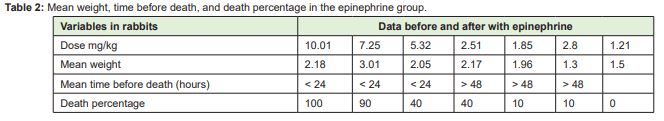
The results for the group receiving lidocaine plus epinephrine are reported in Table 3. In this group, the four rabbits that received 200mg/kg showed drowsiness, lethargy, restlessness, tonic-clonic seizures, difficulty breathing, and nostril flaring, and died, except for one rabbit in this subgroup, which presented all these signs but survived. Both animals receiving 150mg/kg and 125mg/kg showed the same signs and only two died. In the subgroup receiving 100mg/kg, we had to repeat the experiment three times because the first time a rabbit had an adverse reaction and died after 24 hours. In the second attempt, a rabbit died quickly. The third time we conducted the experiment, only one rabbit had a reaction but did not die. The subgroups that received 75mg/kg and 50mg/kg showed no changes.
Although some studies have attempted to demonstrate the safe dose of lidocaine plus epinephrine for subcutaneous administration, the maximum dose has not been determined in the literature.2-5 No experimental studies have been conducted in animals using lidocaine plus epinephrine subcutaneous injection to determine the maximum dose. The recommended safe dose of lidocaine, regardless of the route of administration, is 7mg/kg of body weight.6,19 However, the doses used for subcutaneous injections in liposuction far exceed this value, ranging from 10mg/kg to 75mg/kg.3,4,8-10,12,20,21 These doses have been considered safe in some studies, whereas another study showed some deaths of patients who underwent procedures with injection of high doses of lidocaine. Studies conducted in humans have tried to establish this criterion by increasing the doses of lidocaine combined with other medications.3,9-11
Because liposuction is a very common surgical procedure, we believe that our study contributes to a better understanding and definition of the maximum dose to be used. Our findings show that the use of lidocaine at a dose of 75mg/kg of body weight combined with 0.03mg of epinephrine was a safe dose, causing no external change in the rabbits.
Although the injection of saline and epinephrine is frequently used in liposuction, few experimental studies were found in the literature.15,16 The late deaths (after 48 hours) were not expected because we believed that epinephrine had a short-lasting action.14
Our study conducted in rabbits demonstrates that a dose of 1.21mg/kg of body weight was safe when injected subcutaneously in the abdominal region. Although we are aware that findings from animals cannot be transferred to humans, they indicate a certain correlation, even if indirectly. Even though our study used a simple method, since the data were obtained only by means of clinical observation, the results provide parameters for future research.
Lidocaine injected into the subcutaneous tissue of rabbits at a dose of 50mg/kg did not cause visible reactions or death. The subcutaneous injection of epinephrine in rabbits at a dose of 1.21mg/kg of body weight was shown to be nonlethal. Lidocaine plus epinephrine injected into the subcutaneous tissue of rabbits at a dose of 75mg/kg did not cause visible reactions or death.
All applicable institutional and/or national guidelines for the care and use of animals were followed.
None.
The authors have no financial relationships relevant to this article to disclose.
The authors have no conflicts of interest to disclose.
- 1. Goldstein DS, Golczynska A, Stuhlmuller J, et al. A test of the "epinephrine hypothesis" in humans. Hypertension. 1999;33: 36–43.
- 2. Barone JA, Weisman R, Mangione RA, et al. Serum lidocaine concentrations following subcutaneous administration. Clin Pharm. 1984;3(3):281–284.
- 3. Burk RW, Guzman Stein G, Vasconez LO, Lidocaine and epinephrine levels in tumescent technique liposuction. Plast Reconstr Surg. 1996;97(7):1379–1384.
- 4. Butterwick KJ, Goldman MP, Sriprachya Anunt S. Lidocaine levels during the first two hours of infiltration of dilute anesthetic solution for tumescent liposuction: rapid versus slow delivery. Dermatol Surg. 1999;25(9):681–685.
- 5. Gold MS, Reichling DB, Hampl KF, et al. Lidocaine toxicity in primary afferent neurons from the rat. J Pharmacol Exp Ther. 1998;285(2):413–421.
- 6. Katzung BG. Farmacologia Básica e Clínica. Guanabara Koogan, Rio de Janeiro; 1995.
- 7. Hanke CW, Bernstein G, Bullock S. Safety of tumescent liposuction in 15,336 patients. National survey results. Dermatol Surg. 1995;21(5):459–462.
- 8. Perry AW, Petti C, Rankin M. Lidocaine is not necessary in liposuction.discussion 1903-1906. Plast Reconstr Surg. 1999;104:1900–1902.
- 9. Ostad A, Kageyama N, Moy RL. Tumescent anesthesia with a lidocaine dose of 55mg/kg is safe for liposuction. Dermatol Surg. 1996;22:921–927.
- 10. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248–263.
- 11. Hunstad JP. Tumescent and syringe liposculpture: a logical partnership. Aesthetic Plast Surg. 1995;19:321–333.
- 12. Samdal F, Amland PF, Bugge JF. Plasma lidocaine levels during suction- assisted lipectomy using large doses of dilute lidocaine with epinephrine. Plast Reconstr Surg. 1994;93(6):1217–1223.
- 13. Zhu X, He Q, Lin Z. Monitoring of serum lidocaine concentration in tumescent liposuction infiltrated with high-dosage lidocaine and its clinical significance. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1999;15(1):26–28.
- 14. Goodman L, Gilman LS. As bases farmacológicas da terapêutica. Guanabara Koogan, Rio de Janeiro. 1978.
- 15. Gu X, Simons FE, Simons KJ. Epinephrine absorption after different routes of administration in an animal model. Biopharm Drug Dispos. 1999;20(8):401–405.
- 16. Stein CM, He HB, Wood AJ. Basal and stimulated sympathetic responses after epinephrine: no evidence of augmented responses. Hypertension. 1998;32(6):1016–1021.
- 17. McCullagh P, Nelder JA. Generalized linear models. Chapman and Hall, London; 1989.
- 18. SAS Institute Inc. SAS/STAT User’s Guide, Version 8. SAS Institute, Cary; 2000.
- 19. Melega JM, Cirurgia Plástica: fundamentos e arte. Editora Médica e Científica Ltda, Rio de Janeiro; 2002.
- 20. Rao RB, Ely SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(9):1471–1475.
- 21. Matarasso A. Lidocaine in ultrasound-assisted lipoplasty. Clin Plast Surg. 1999;26: 431–439,viii-ix.

