Background: Breast reconstruction surgery is an exceptionally important tool for repair of defects and asymmetries caused by the various types of surgical breast cancer treatment. Reconstruction is indicated regardless of lesion location in the breast when a quadrantectomy or sector resection has been performed. Although several surgical techniques are available for reconstruction, we propose a method based on contralateral breast graft replacement to preserve shape and symmetry.
Methods: This quasi-experimental study was performed with a sample of 42 women who had undergone quadrantectomy or wide resection, followed by radiation therapy, for treatment of breast cancer. The outcomes of interest were breast aesthetics and symmetry in the immediate postoperative period and after 3 months of radiation therapy. All patients were photographed preoperatively, pre-radiation therapy, and 3 months post radiation therapy. Before-and-after photographs were sent to four independent experts in the field, who, in a blinded manner, used a continuous visual analogue scale (VAS) of 0 to 10 to assess breast symmetry and aesthetic appearance. Results: The mean (SD) VAS score was 8.74 (0.75) for symmetry and 8.76 (0.80) for aesthetics (p>0.50 for both). Comparison of VAS scores assigned to preoperative and postoperative images revealed that autologous breast tissue grafting was useful.
Conclusion: These findings suggest that, when combined with established techniques, autologous contralateral breast tissue grafting is a feasible alternative for breast reconstruction that provides satisfactory aesthetics and symmetry.
Keywords: Autologous breast grafting, Breast reconstruction, Breast surgery
Early diagnosis and treatment of breast cancer is associated with higher rates of cure, consequently reducing the negative impact on body image and stigma brought by the disease.1 It is noteworthy that an extensive body of clinical research has demonstrated that the outcomes of radical, mutilating treatments for breast cancer are similar to those provided by breast-conserving therapy, with equivalent prognosis and survival according to the stage at diagnosis and histological tumor type.2,3 The radical mastectomy procedure developed by Halsted was long the standard for surgical treatment of breast cancer. In 1930, Patey and Handley proposed a modified technique that spared the pectoral muscles, and, in 1994, Veronesi proposed quadrantectomy and sector resection combined with adjuvant treatments such as chemotherapy and radiation therapy.4 Several techniques have since been developed to reduce the damage caused by surgical treatment of breast cancer. Among these, those most widely recommended are the transverse rectus abdominis myocutaneous (TRAM) flap and the deep inferior epigastric perforator (DIEP) flap.
The TRAM flap, first described by Drever5 and subsequently modified by Hartrampf, et al. and Gandolfo in 1982,6-8 allows translation of skin, fat, and abdominal muscle to the anterior chest region, using the superior epigastric vascular pedicle, which tracks the thickness of the anterior rectus abdominis muscles. The latissimus dorsi flap technique involves partial transposition of the latissimus dorsi muscle along with a skin and subcutaneous tissue island of adequate size. First described by Tansini in 19069 and later modified by Bostwick in 197810, this procedure depends on the integrity of the thoracodorsal neurovascular pedicle. It is usually accompanied by placement of an implant or tissue expander to ensure appropriate volume. Indeed, silicone implants or expanders have been an excellentchoice for breast reconstruction. Both can be used in conjunction with other techniques such as the TRAM and latissimus dorsi flaps, and silicone implants can also be used separately, to fill out small imperfections.
The DIEP is similar to the TRAM flap, except that no muscle is used to reconstruct the breast, whereas the TRAM uses one or both rectus abdominis muscles. As its name implies, the DIEP uses the deep inferior epigastric perforator arteries, and no muscle is cut or removed. This technique requires special training and experience in microsurgery, an ability that many surgeons lack.11,12
Grafting refers to the transplantation of a tissue or organ completely isolated from the donor – and thus lacking a vascular pedicle or venous supply – to a recipient. In an autologous graft, or autograft, the donor and recipient are the same individual. The first autografts performed were skin grafts, attempted in the 1800s by Paronio. Soon after, in 1823, von Gräfe reported a nose graft using skin taken from the thigh of the patient, and in 1840, in Boston, Warren performed a full-thickness autologous skin graft to the ala nasi. Larger, thicker skin grafts were soon attempted by the likes of Ollier in 1872 and Thiersch in 1874. Lawson (1870), Le Fort (1872), and, especially, Wolfe (1876) used full-thickness skin grafts for the treatment of lower eyelid ectropion. In 1929, the development of special knives by Blair and Brown and the invention of the mechanical dermatome by Padgett were followed by a huge increase in the use of grafts. This led to rapid development of other tissue grafts, such as cartilage, bone, fat, tendon, and fascia lata, among several others).13-19 The use of autologous breast tissue, however, is rarely mentioned in the literature. Most publications have focused on the use of autologous fat grafting and pedicled autografts, usually involving microsurgery.7,8,11,12 Additional studies are needed to assess the clinical outcomes of autologous breast tissue grafting. Within thiscontext, the aim of the present study was to evaluate the results of a contralateral breast tissue graft combined with other, established surgical techniques for breast reconstruction. Outcomes were assessed by blind expert evaluation of breast aesthetics and symmetry in the immediate postoperative period and after 3 months of radiation therapy.
This study was approved by the Hospital de Clínicas de Porto Alegre (HCPA) Research Ethics Committee (protocol no. 11-0013). According to the Declaration of Helsinki, all patients provided written informed consent for participation.
Design overview, setting, and participants
A quasi-experimental study was performed at private hospitals in Porto Alegre, Brazil, between 2012 and 2015. The participants were women aged 28-80 years who had received a diagnosis of breast cancer with indications for breast- conserving surgery and had been referred by specialist breast surgeons in Porto Alegre. No patient had received any previous radiation therapy, but neoadjuvant chemotherapy was allowed.
The final sample comprised 42 patients who had undergone surgery over a 2½-year period. All patients were evaluated before and after surgery and followed for at least 3 months after radiation therapy, which lasted 6 weeks, as is standard practice.20
Once participation in the study had been defined, patients were informed of the intended therapeutic method (breast reconstruction surgery combined with an autologous contralateral breast tissue grafting technique). All patients had indicationsfor breast-conserving surgery, as determined previously by their breast cancer specialists. No patient had undergone previous cancer surgery and all wished to undergo breast reconstruction. Patients with a history of prior surgery or radiation therapy, as well as patients with indications for mastectomy, were excluded from the study. The sequence of assessments is presented in Figure 1.
Assessment
The dependent variables of interest were breast symmetry and aesthetics as assessed on a visual analogue scale (VAS) of 0 to 10, with 0 denoting a poor result and 10, an excellent result. The scale consisted of a 10-cm rule with millimeter markings. Patients were not identified on any images.
Four expert members of the Brazilian Society of Plastic Surgery were chosen as raters. Each rater received a booklet containing 43 preoperative and postoperative images and a statement instructing the rater to evaluate the images for breast symmetry and aesthetics and grade these parameters by marking a horizontal line in ink on the VAS below each image. The booklet noted that all patients had undergone breast reconstruction after breast-conserving cancer surgery and were 3 months post- radiation therapy. The authors of the study and the raters did not know each other, and the person who distributed the questionnaires was unaware of their content. The study was thus blinded with respect to ratings and distribution. All four raters completed the questionnaire, and their scores were analyzed statistically.
Procedures
All patients were photographed with a 10-megapixel digital camera (Canon) in automatic mode. Frontal and right and left oblique photographs were obtained with patients in the standing position, 1 m away from the camera. Preoperative marking was performed with patients in the standing position, and always followed established practice for the chosen breast mammaplasty technique. The techniques used were those described by Pitanguy21, McKissock22, Ribeiro23, and Georgiade.24
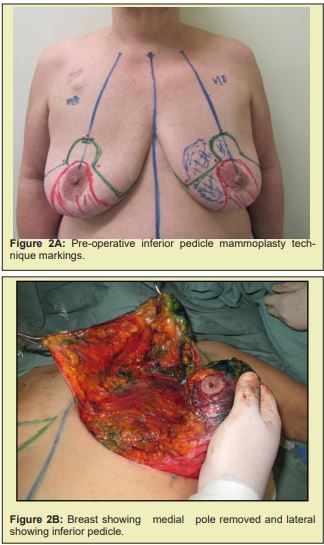
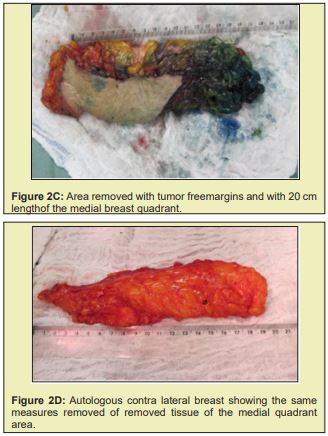
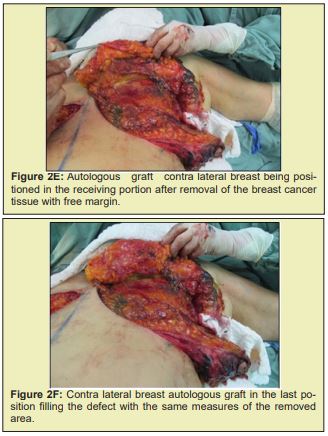
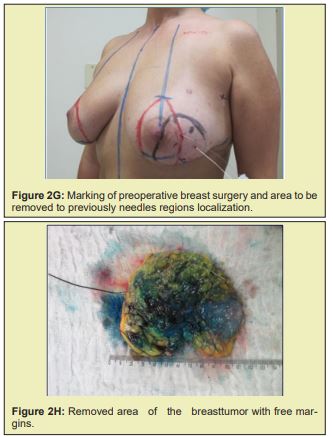
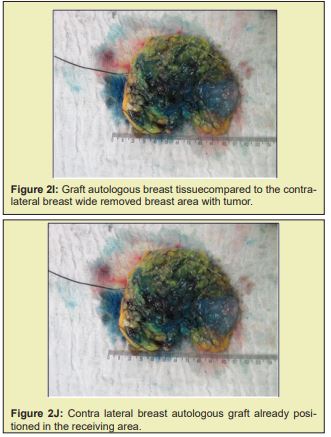
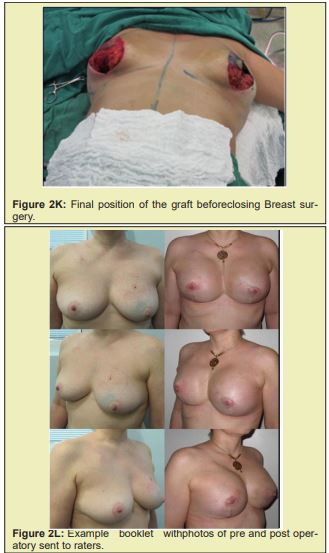
Briefly, patients were positioned supine with the arms abducted 90° and secured by a strap to a clamp attached to the operating table. The anterior and lateral cervical and thoracic regions were prepped and skin antisepsis performed with chlorhexidine gluconate in alcohol solution. The operative field was bordered by sterile drapes, held in place with towel clamps so as to leave only the prepared skin area exposed. In some patients, mammography-guided needle-wire localization had been performed preoperatively, at the breast cancer specialist’s discretion. The initial approach was performed in accordance with the chosen breast reduction technique. Both breasts were exposed simultaneously. The target area for resection in the diseased breast, with sufficient margins, was identified by a pathologist in the operating room. The tumor mass was then removed, exposing the defect to be filled by the graft. Electrocautery use was kept to a minimum to prevent necrosis of defect margins. The resected specimen was then measured, the defect area secured, and a piece of tissue of the same size and shape removed from the contralateral breast. The resulting tissue graft was then positioned and affixed with simple interrupted sutures (nylon 3-0). This was done immediately after graft removal, to minimize time withoutvascularization and without contact with the new breast tissue. After this step, the surgery was continued according to the technique chosen for each procedure Figure 2.21-25
To reduce assessment bias, the raters were blinded to study authorship as noted above. The raters were selected among members of the Brazilian Society of Plastic Surgery active in the scientific department of this society. All patients underwent clinical evaluation and all surgeries were performed by the same operator, who had many years of experience in breast cancer surgery.
Statistical analysis
Descriptive statistics were used to summarize the main characteristics of the sample. The 90thand 10thpercentiles were selected as the cutoffs for ceiling and floor effects respectively. The t-test for independent samples was used to compare continuous variables. Kendall’s W statistic was used to assess agreement among raters regarding the symmetry and aesthetics parameters. W coefficient values range from 0 to 1; the closer to 1, the better the agreement. The significance level was set at p<0.05(two-tailed) for all analyses. Data were analyzed using SPSS version 22 (IBM Corp., Armonk, NY).
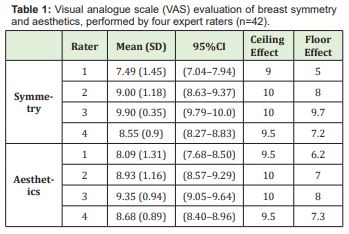
The mean patient age was 51.91 years (SD =11.00). Before-and-after images (obtained preoperatively and 3 months after radiation therapy) were assessed for symmetry and aesthetics by four independent raters. The mean VAS scores assigned by the raters are presented in Table 1. Complications, which progressed to fibrosis and partial fat necrosis after 3 months of radiation therapy, were observed in 10 cases. However, these cases were easily addressed by performing subcision via a small incision made with a #11 scalpel blade. A comparative analysis of symmetry and aesthetics outcomes for these 10 patients versus those who did not experience complications (n=32) was carried out. Mean symmetry scores were significantly lower among patients with complications (8.10 [0.85] vs. 8.93 [0.60] for the non-complication group, p=0.001), as were aesthetics scores (7.83 [0.55] vs. 9.05 [0.63] for the non-complication group, p<0.001).
Scores and their ranges (minimum-maximum) are presented in Table 2. There was a minimal ceiling effect from the highest possible VAS score. Ceiling and floor effects were assessed to demonstrate that raters’ scores were distributed within the range of the defined scale (0-10). Inter-rater agreement was moderate for both symmetry and aesthetic criteria (Kendall’s W, 0.55 for symmetry, p<0.001; 0.60 for aesthetics, p<0.001).
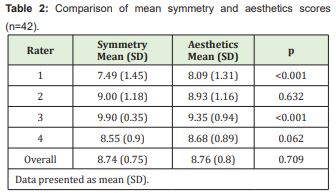
The scores assigned by the four raters were averaged to yield an overall score. Overall symmetry and aesthetics scores were then compared using the paired t-test (Table 2). Rater 1 was found to assign significantly lower average scores for symmetry than for aesthetics, whereas rater 3 assigned significantly higher average scores for symmetry than for aesthetics.
A VAS score of 8 was defined as the cutoff point for good/excellent outcome. As shown in Table 3, average scores for symmetry and aesthetics were significantly higher than 8, except those assigned by the first rater.
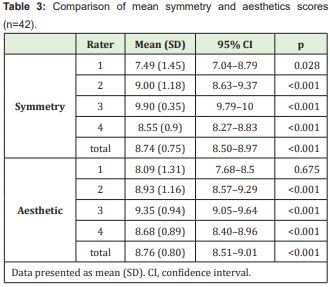
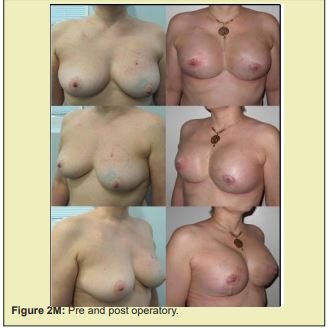
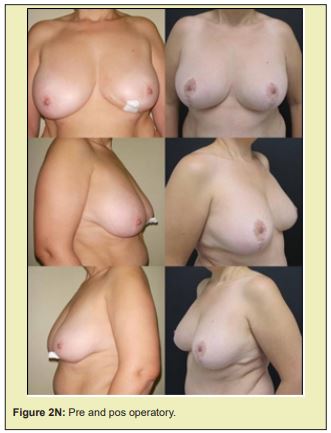
Figure 2 shows a selection of before-and-after images from illustrative cases to demonstrate the symmetry and aesthetics outcomes achieved with the proposed technique (2M to 2R).
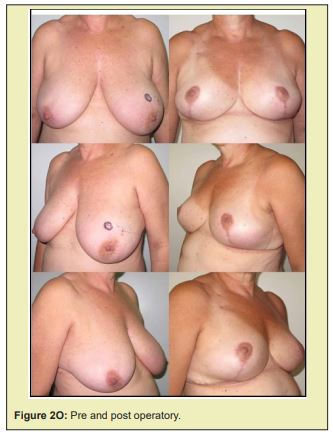
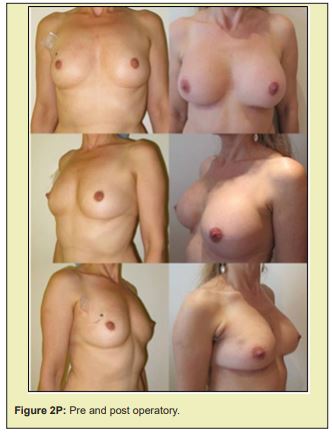
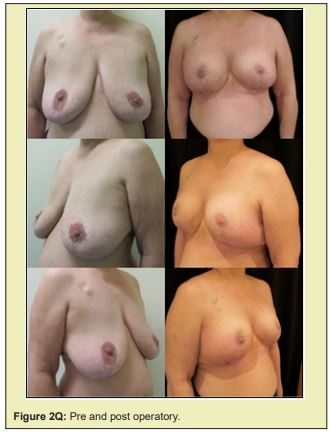
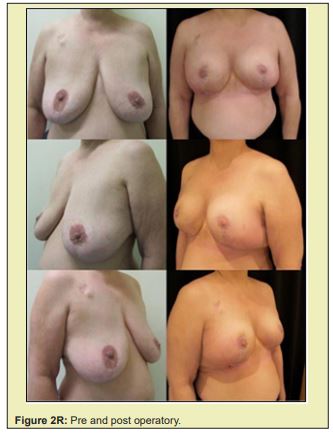
Based on expert assessment of breast aesthetics and symmetry parameters, the contralateral breast tissue autograft technique proposed in this study produced satisfactory results. The mean overall symmetry and aesthetics scores exceeded the defined cut off point of 8. Ceiling and floor effects were observed in less than 10% of scores. Additionally, the complications observed can be considered minor, and were corrected without serious consequences.
Taking into account that, according to World Health Organization data, approximately 1,050,000 new cases of breast cancer occur worldwide each year, the proposed technique has the potential for substantial clinical relevance. Although many methods are available for breast reconstruction with good results,5-7 this new approach may be especially useful in patients undergoing breast-conserving surgery, in which a large part of the breast is usually removed. As the amount of tissue removed plays an essential role in achieving local tumor control and sufficient clear margins must be maintained to ensure adjacent tissues are free of disease, a significant volume of breast tissue may be removed. Hence, breast reconstruction is mandatory to prevent the physical and emotional sequelae of breast cancer surgery.26-30 One important point to be considered is that some established techniques31,32 actually make the lack of tissue more evident, especially when performed in smaller breasts.33,34 Furthermore, preservation of breast aesthetics and symmetry is sometimes impossible with current single-stage procedures.
Many surgical techniques have been proposed for breast reconstruction, such as the TRAM, latissimus dorsi, DIEP, and a variety of local flaps.7,10,11,35 All produce aesthetically satisfactory results in many cases; however, indications for these techniques are sometimes limited by the lack of a suitable donor area.5-7
Hence, alternative breast reconstruction techniques with potential economic benefits and providing a satisfactory degree of aesthetics and symmetry are required. To bridge this gap, autologous fat grafting as proposed in the present study might improve the contour of the reconstructed breast, when combined with other techniques, and/or improve skin quality in retractions caused by radiation therapy. Fat grafting is contraindicated for correction of vast defects, because large empty spaces are known to increase the risk of necrosis.36,37 In these situations, we decided to use healthy contralateral breast tissue to simulate the size and volume of the resected tissue and thus restore the defect. The proposed technique is supported by a long history of success with autologous grafting of other tissues, such as cartilage, bone, fat, and skin.13-16 However, long-term observation is needed before consistent, definitive conclusions can be reached. One must also consider that the effect of radiation therapy on contralateral breast tissue autografts is unknown. Only long-term follow-up can provide the necessary information to answer these questions.
Of the 42 patients assessed after 3 months of radiation therapy in this study, 10 (23.8%) had developed hardening (fibrosis) and partial fat necrosis. However, these cases were readily addressed by subcision (subcutaneous incisionless surgery), a technique first described in 1995 to manage deep wrinkles and depressions in the face.38
This simple procedure is often used in dermatology to treat cellulite by improving tissue distribution and fibro-glandular symmetry and consistency. It should be stressed that symmetry and aesthetics were not compromised in these 10 patients. Overall, these findings suggest that the technique proposed in this study is safe and yields satisfactory results.
To the best of our knowledge, this is a novel approach and has not been described previously; thus, there are no prior studies to compare with the presentfindings. It is important to note that the aim of this study was to evaluate aesthetics and symmetry after 3 months of radiation therapy. Outcomes were evaluated by four specialists who had no knowledge of the indications for surgery or of the technique used for breast reconstruction. This approach was used to ensure that raters would assess symmetry and aesthetics in a blinded manner. However, further studies with larger sample sizes and longer follow-up periods are needed. It is important to consider that the findings of this study suggest an alternative technique that could be used to reduce mutilation consequent to breast cancer surgery. As demonstrated by the range of VAS scores (0-10), there was some variation in raters’ assessments, but most scores were 8 or higher. The absence of a high concentration of symmetry or aesthetics scores near the upper or lower limits indicates that raters’ measures were valid and reliable. Furthermore, Kendall’s W coefficients showed only a small difference between raters.
An important point to consider in the context of surgical techniques is that results are strongly associated with patient characteristics; many potential confounding factors cannot be adequately controlled, since they are inherent to surgical techniques and individual patient profiles. Within this context, the single- subject design used in the present study allowed patients to serve as their own controls. This design is also sensitive to individual differences, and allows assessment of causal relationships between the independent variable (in this case, surgical technique) and dependent variables (symmetry and aesthetics).39 While it reduces the potential for comparison with other patients, it is an ideal strategy to validate results, because in real-life settings, patients have different profiles and it is impossible to monitor the impact of factors, such as genetic, immunological, and emotional aspects, which could have a direct influence on the outcomes of interest.
One could argue that the non-randomized design is a limitation of the present study. However, this design has the advantage of avoiding possible data contamination, which can counterbalance the limitation imposed by the absence of randomization. Considering that breast cancer surgery is the most prevalent in females, these results have greater clinical applicability. Treating breast cancer with a single-stage surgical procedure with low potential for morbidity may allow patients to resume their daily activities immediately. These are potential advantages that need to be discussed in the clinical decision-making process, in which patients should also participate. Finally, some concerns that could be addressed in future research include the therapeutic implications of this transplanted tissue and immunohistochemical studies to measure vascular endothelial growth factor (VEGF).
The use of autologous tissue from the contralateral breast for reconstruction after breast-conserving surgery, combined with other mammaplasty techniques, proved to be a valid alternative providing acceptable symmetry and breast aesthetics, as rated by blinded experts.
None.
National Council for Scientific and Technological Development -CNPq (W. Caumo); Graduate Program in Medical Sciences, School of Medicine, Universidade Federal do Rio Grande do Sul.
The authors declare that there are no financial or other relationships that might lead to conflicts of interest involving any of the following arrangements: financial relationship to the work, employees of a company, consultants for a company, stockholders of the company, members of a speakers bureau or any other form of financial compensation.
- 1. Ely S, Vioral AN. Breast cancer overview. PlastSurgNurs. 2007;27(3):128–133.
- 2. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410.
- 3. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41(3A):151–152, discussion 2-3.
- 4. Veronesi U. Quadrantectomia. In: Bland KI, Copeland EM. Editors. A mama: Tratamentocompreensivo das doençasbenignas e malignas. São Paulo: Manoele Ltda, 1994, p. 715–717.
- 5. Drever JM. Total breast reconstruction with either of two abdominal flaps. PlastReconstr Surg. 1977;59(2):185–190.
- 6. Hartrampf CR, Scheflan M, Black PW. Breast reconstruction whit a transverse abdominal island flap: A retrospective evaluation of 355 patients. Plast Reconstr Surg. 1987;(1):123–127.
- 7. Teymouri H, Stergioula S, Eder M, et al. Breast reconstruction with a lower abdominal myocutaneous flap. Br J Plast Surg. 1982;25:452–457.
- 8. Hartrampf CR Jr, Bennett GK. Autogenous tissue reconstruction in the mastectomy patient: a critical review of 300 patients. Ann Surg. 1987;205(5):508–519.
- 9. Tansini I. Sopra il mio nuovo processor di amputazionedellamammella. (coverage of the anterior chest wall following mastectomy). Guz Mal Ital.1906;57:141.
- 10. Bostwick J, Vasconez LO, Jurkiewicz MJ. Breast reconstruction after a radical mastectomy. PlastReconstr Surg. 1978;61(5):682–693.
- 11. Hamdi M, Weiler Mithoff EM, Webster MH. Deep inferior epigastric perforator flap in breast reconstruction: experience with the first 50 flaps. PlastReconstr Surg. 1999;103(1):86–95.
- 12. Bodin F, Dissaux C, Lutz JC, et al. The DIEP flap breast reconstruction: Starting from scratch in a university hospital. Ann ChirPlast Esthet. 2015;60(3):171–178.
- 13. Jansen C. Cartilage tympanoplasty. Laryngoscope.1963;73:1288–1301.
- 14. Dimitriou R, Mataliotakis GI, Angoules AG, et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injured. 2011;42(2):S3–S15.
- 15. Coleman SR. Structural fat grafting: more than a permanent filler. PlastReconstr Surg. 2006;118(3 Suppl):108S–20S.
- 16. Illouz YG. The fat cell “graft”: a new technique to fill depressions. PlastReconstr Surg. 1986;78(1):122–123.
- 17. Millar R, Dickie WR, Colville J. The results of long delayed flexor tendon graft. Hand. 1972;(3):261–262.
- 18. Moberg E. Experiences with Bunnell’s pull-out wire sutures. Br J Plast Surg.1951;38:175.
- 19. Neuhof H. Fáscia transplantation into visceral defects: an experimental and clinical study. Surg Gynecol Obstet. 1917;24:383–427.
- 20. Mansfield C. Effects of radiation therapy on wound healing after mastectomy. Clin Plast Surg. 1979;6(1):19–26.
- 21. Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg. 1967;20(1):78–85.
- 22. McKissock PK. Reduction mamaplasty with a vertical dermal flap.PlastReconstr Surg. 1972;49(3):245.
- 23. Ribeiro L. Cirurgiaplástica da mama. Rio de Janeiro: Medsi. 1989.
- 24. Georgiade GS, Riefkohl RE, Georgiade NG. The inferior dermalpyramidal type breast reduction: long-term evaluation. Ann Plast Surg. 1989:23(3):203–211.
- 25. Courtiss EH, Goldwyn RM. Reduction mammaplasty by the inferior pedicle technique. An alternative to free nipple and areola grafting for severe macromastia or extreme ptosis. PlastReconstr Surg. 1977;59(4):500–507.
- 26. Kübler Ross E (ed.). O que osdoentesterminaistêm para ensinar a médicos, enfermeiras, religiosos e aosseusprópriosparentes. Trad Paulo Menezes. São Paulo: Martins Fontes, 1994.
- 27. Parkes CM. Estudossobre a perdanavidaadulta. Trad. Maria Helena Franco Bromberg. São Paulo: Summus, 1998.
- 28. Dian D, Schwenn K, Mylonas I, et al. Quality of life among breast cancer patients undergoing autologous breast reconstruction versus breast conserving therapy. J Cancer Res Clin Oncol. 2007;133(4):247–252.
- 29. Al-Ghazal SK, Fallowfield L, Blamey RW. Comparison of psychological aspects, and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer. 2000;36(15):1938–1943.
- 30. Veiga DF, Veiga Filho J, Ribeiro LM, et al. Evaluations of aesthetic outcomes of oncoplastic surgery by surgeons of different gender and specialty: a prospective controlled study. Breast. 2011;20:407–412.
- 31. Veronesi U, Cascinelli N, Mariani L, et al. Twenty year follow up of a randomized study comparing breast- conserving surgery with radicalmastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232.
- 32. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241.
- 33. Iwuchukwu OC, Harvey JR, Dordea M, et al. The role of oncoplastic therapeutic mammoplasty in breast cancer surgery - a review. Surg Oncol. 2012;21:133–141.
- 34. Giacalone PL, Roger P, Dubon O, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2007;14:605–614.
- 35. Holmström H, Lossing C. The lateral thoracodorsal flap in breast reconstruction. PlastReconstr Surg. 1986;77(6):933–943.
- 36. Choi MS, Levovitz C, Lee C, et al. The volumetric analylis of fat graft survival in breast reconstruction. Plastic Recontr Surg. 2013;131(2):185.
- 37. Missana MC, Laurent I, Barreau L, et al. Autologous fat transfer in reconstructive breast surgery:indications, technique and results. Eur J SurgOnco. 2007;33(6):685–690.
- 38. Orentreich D. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrink. Dermatol Surg. 1995;21(6):543–549.
- 39. Dallery J, Cassidy RN, Raiff BR. Single-case experimental designs to evaluate novel technology-based health interventions. J Med Internet Res. 2013;15:e22.

