Most studies agree on the demonstration of the existence of nerve tissue specificity and that if a non-nervous target (e.g. a tendon) were sutured to one of the distal limbs of a Y-shaped nerve guide, independent of the type of guide, all nerve fibers would regenerate toward the nerve tissue. Recent studies suggest that optimum nerve regeneration may arise from the tissue environment in general. We developed an experimental model in order to eliminate all the microenvironment problems that we experienced in previous studies, and to be able to evaluate the neurotropic affinity of various tissues.
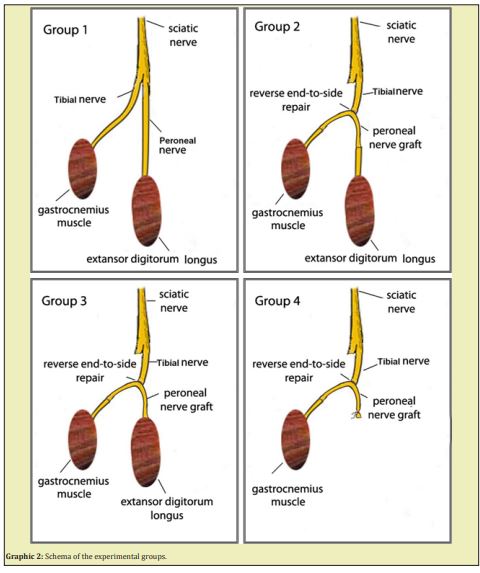
Group 1 (n:8): The control group. The sciatic nerve was only explored, but certainly not touched.
Group 2 (n:8): Both lower ends of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, were coapted to tibial and peroneal nerves distally by end-to-end coaptation.
Group 3 (n:8): As one end of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, was coapted to the peroneal nerve distally, the other end was sutured directly by opening the fascia of the gastrocnemius muscle.
Group 4 (n:8): As one end of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, was coapted to peroneal nerve distally, the other end was ligated
As seen in the histomorphometric analysis, axons were observed to sprout towards both ends of the horseshoe-shaped nerve graft in a free fashion without being affected by the negative influences of the microenvironment.
Keywords: Histomorphometric analysis, Axons
Neurons are dependent on neurotrophic factors produced by the targets. The target produces neurotrophic factors, which are taken up by the axon terminals and transported retrogradely to the nerve-cell body to support its normal metabolic processes. At the level of axonal transactions, new axonal sprouts begin to grow distally. Schwann cells at the site of injury start to produce neurotrophic factors supplied locally by Schwann cells. Neurotrophic factors support the survival and general growth capacities of neurons. They are normally present at very low levels in the non-neural cells of a nerve trunk, but a local injury will initiate a local synthesis at relatively high levels.1 Enclosure of the injury zone in a tube, leaving a gap between the ends, may contribute to local accumulation of various factors, resulting in the optimal statement of trophic and tropic mechanisms.2
The existence of the specificity of peripheral nerve fiber regeneration has been investigated at two levels. The first level specificity is “tissue specificity”, which can be defined as the preferential re-innervation of distal nerve tissue versus other types of tissue. Most studies agree on demonstrating that there is nerve tissue specificity and that if a non-nervous target (e.g., a tendon) were sutured to one of the distal limbs of a Y-shaped nerve guide, independent of the type of guide, all nerve fibers would regenerate toward the nerve tissue. In contrast, there is much less agreement regarding the presence and features of a second level of specificity, i.e., the possibility that peripheral nerve fiber regeneration is not only guided by simple nerve tissue specificity, but that it also responds to higher and more discriminative types of distal lures within the nerve tissue.2-4 The concept that the distal stump of a transected nerve attracts regenerating axons, has had a chequered career. This was originally avowed by Forssman and Cajal.5,6
Weiss and Taylor then re-investigated the question by in vivo experiments in which the proximal end of the divided sciatic nerve in the rat was inserted into a Y-shaped tube fashioned from the distal aorta from another rat, and its bifurcation into the two iliac arteries. This offered the regenerating axons the choice of growth into one or other of the iliac arteries. If the distal stump of the sciatic nerve were inserted into one of the distal limbs of the graft, the other was left empty, ligated or plugged. Weiss and Taylor therefore concluded that the distal stump had no attractive effect on regenerating axons.7 Weiss later failed to find evidence of neurotropism in tissue culture experiments and showed that alteration of the substrate surface influenced the direction of neurite outgrowth.8 The concept of a neurotropic influence on nerve regeneration was never completely discarded. It was restored to respectability by Politis, et al. who produced an in vivo demonstration of a neurotropic influence from the distal stump on regenerating axons using a Y-shaped silicone tube, and Anderson & Turmaine demonstrated why Weiss's experiment had failed. Anderson & Turmaine hypothesized that the grafted aorta may have become semipermeable.9-11 At about the same time, Lundborg & Hansson found that peripheral axons regenerating through mesothelial chambers would cross to reach the distal stump of the nerve at the opposite corner of the chamber.12 A neurotropic influence of the distal stump on regenerating axons was confirmed by Ochii, and in primates, by MacKinnon et al. and Nachemson & Lundborg.13-15
Classical studies have shown that diffusible factors from the distal stumps of transected peripheral nerves exert a eurotropic/chemiotactic effect on regenerating nerve fibers in vivo.16-18,2,6 Recent studies suggest that optimum nerve regeneration may arise from the tissue environment in general.19
The various levels of specificity of regenerating peripheral nerve fibers have been addressed by a number of studies. Several authors investigated the tissue specificity in vivo, adopting slightly different models. These included a Y-shaped artery obtained from heterogenic or syngeneic animals and Y-shaped pre-degenerated muscle.7,11,20 In particular, tissue specificity was usually investigated by the Y-chamber experimental model.7,9,10,11,14,20-27
Autologous nerve grafting is still considered the best method for bridging peripheral nerve injuries with substance defects. A regenerating nerve should exhibit axonal growth and extension. These events are of multifactorial character for which the ideal microenvironment is a nervous tissue. Therefore, using nerve grafts is also the best method for bridging nerve defects. The Schwann cell and its basal lamina act as a template in this process.28-32 The serious problems of using non-absorbable materials are complications such as local fibrosis or mechanical discomfort.33 The demonstration that the peripheral nerve microenvironment will allow regeneration of central nervous tissue as well as peripheral axons has stimulated significant interest in and brought talent to the study of peripheral nerve regeneration.34
The microenvironment of the tip of the regenerating peripheral nerve is a major focus of contemporary research into methods of enhancing peripheral nerve regeneration. Progress toward isolating new growth-promoting agents has been accelerated by in vitro cell culture methods and more recently by the nerve guide or the nerve regeneration chamber model in which agents can be tested for the ability to promote nerve regeneration in vivo. The nerve guide or nerve regeneration chamber model is an in vivo surgical preparation in which we may observe, sample and manipulate the microenvironment of the regenerating peripheral nerve.32
The models prepared from non-neural tissues show limited success in the evaluation of neurotropism due to failure to obtain an appropriate microenvironment and occurrence of foreign body reactions.35 A nerve can regenerate itself best within a nervous tissue.32 Therefore, we created a model similar to Y-tube by nervous tissue using a combination of reverse end-to-side coaptation technique and nervous graft. I had introduced this model in my article titled as “A Successful Neurotization of Two Different Muscles Using a Single Intact Motor Nerve: Experimental Study on Rats” and had noted that horseshoe shaped nerve graft showed a better axonal extension towards both of the legs compared to the Y-shaped grafts.36-41 In light of this study, we developed an experimental model in order to eliminate all of the microenvironment problems that we experienced in the previous studies, and to be able to evaluate the neurotropic affinity of various tissues.
Thirty-two adult female Sprague-Dawley rats weighting 300-350gr. were used in this study. Each was maintained under standard laboratory conditions. The rats were allowed free access to rat chow and water. They were randomly and equally divided into four groups. General anesthesia was accomplished by an intraperitoneal injection of a mixture of ketamine (90mg/kg), xylazine (10mg/kg). The animals were placed in the prone position on the operating table with the lower extremities in extension. A posterior incision, starting from the iliac bone spine, reaching the lateral condyle of knee, was used for exposure. The leg muscles, the sciatic nerve and its branches were exposed. A 2cm peroneal nerve graft was transected, and the tibial nerve was transected. The intact donor nerve end was sutured to the epineural window at the middle of the horseshoe shaped nerve graft and one end of the nerve graft was coapted to the tibial nerve, and the other end was coapted to different tissues according to groups. All neurological surgeries were performed carefully under x25 magnification and with 11/0 prolene sutures (Graphic 1).
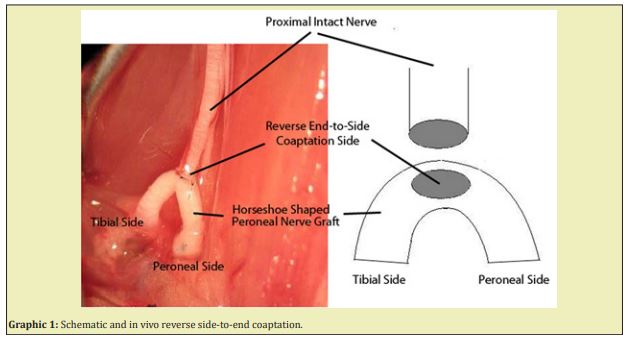
Group 1 (n:8): The control group. The sciatic nerve was only explored, but certainly not touched.
Group 2 (n:8): Both lower ends of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, were coapted to the tibial and peroneal nerves distally by end-to-end coaptation.
Group 3 (n:8): As one end of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, was coapted to the tibial nerve distally, the other end was sutured directly by opening the fascia of the extensor digitorum longus muscle.
Group 4 (n:8): As one end of the peroneal nerve graft, prepared by reverse end-to-side coaptation in the shape of a horseshoe, was coapted to tibial nerve distally, the other end was ligated.
(Graphic 2).
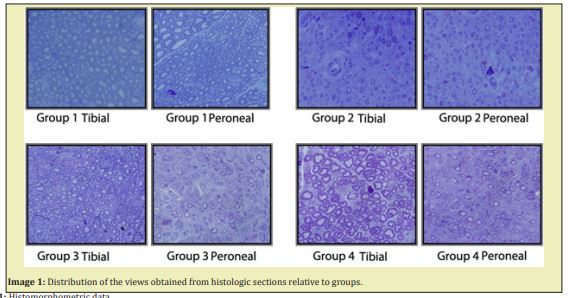
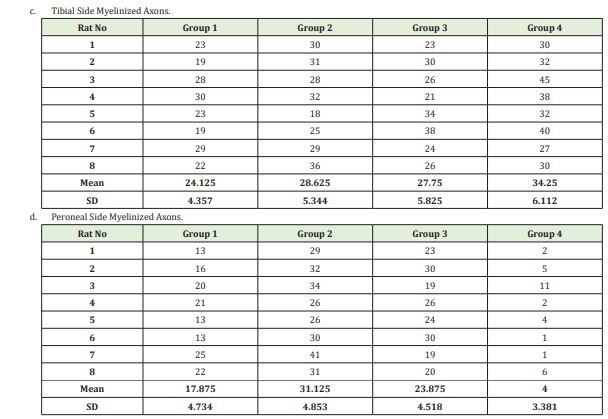
All rats were sacrificed after 12 weeks, and specimens were obtained to be evaluated under light microscopy. The specimens were collected from both the peroneal and the tibial sides of the nerve grafts and from the proximal end of the tibial nerve, which is a source of new axons. They were stained with 0.5% toluidine blue for light microscopy to evaluate myelinated and unmyelinated axons. Photographs at standard size from 5 different regions of each specimen were randomly taken during all microscopic examinations. The mean value for each specimen was obtained after the number and thickness of axons were calculated on the photographs.
Statistical calculations were performed using the One Way Anova (Holm Sidak Method) test and the differences with p, 0.05 were considered significant.
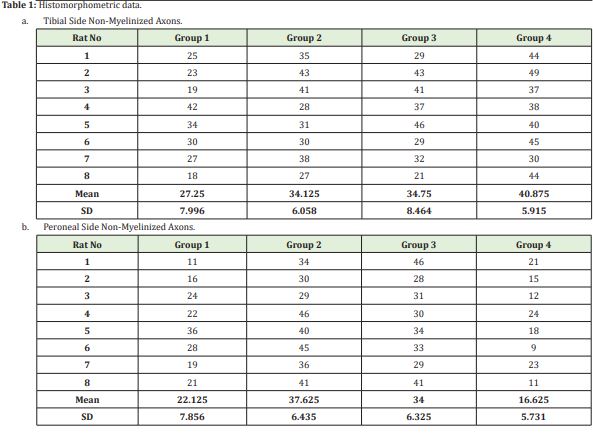
The number of non-myelinated axons
Having determined at least one different group relative to the number of tibial non-myelinated axons using the One-way ANOVA (Holm Sidak Method) test (p=0.08), we carried out inter-group comparisons. In these analyses, Group 1 showed no significant difference compared to Group 2 and Group 3. However, Group 4 was found to exhibit a statistically significant difference compared to Group 2 (p=0,041) and Group 3 (p=0,002). There was no difference between Group 3 and Group 4 (p=0,116).
After having determined at least one different group relative to the number of peroneal non-myelinated axons by the One-way ANOVA (Holm Sidak Method) test (P≤0.001), we carried out inter-group comparisons. There was no statistically significant difference between Group 1 and Group 4 (p=0.124). Group 2 and Group 3 did not demonstrate a difference either (p=0.275). The other inter-group comparisons revealed differences in terms of mean values.
The number of myelinated axons
Having determined at least one different group relative to the number of tibial non-myelinated axons using the One-way ANOVA (Holm Sidak Method) test (P=0,009), we carried out inter-group comparisons. Group 1 and Group 4 demonstrated a statistically significant difference (P=0,002). No other statistical difference was observed between the study groups.
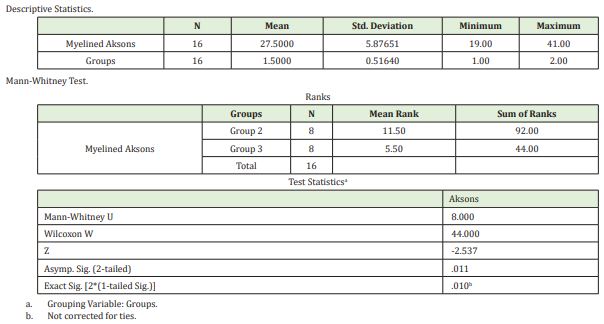
Having determined at least one different group relative to the number of peroneal non-myelinated axons using the One-way ANOVA (Holm Sidak Method) test (P≤0,001), we carried out inter-group comparisons. There were statistically significant differences between all the groups. As proposed in our experimental study, more myelinated axons were expected to be formed in Group 2 peroneal legs of our model compared to the myelinated nerves in the peroneal legs of Group 3. This was shown statistically when these two groups were compared with the Mann Whitney U test and the p value was found to be 0.011 (<0.005); namely, more myelinated axons were formed in Group 2, as expected(Table 1, Image 1)
The study of peripheral nerve regeneration has always attracted the interest of researchers due to its many peculiar features differentiating it from the regenerative processes of all other tissues.30,36-38 Among the different aspects of nerve regeneration, the issue of the specificity of peripheral nerve regeneration has been particularly debated over the years. Specificity of nerve regeneration can be defined as the ability of the nerve fibers of a peripheral nerve, after a lesion, to regenerate preferentially back to its original target.4 Enclosure of the injury zone in a tube leaving a gap between the ends, may contribute to local accumulation of various factors, resulting in the optimal statement of trophic and tropic mechanisms.2 The various levels of specificity of regenerating peripheral nerve fibers have been addressed by a number of studies. In particular, tissue specificity was usually investigated by the Y-chamber experimental model.7,10,11,14,20-24 It may be concluded that tropic factors that exert chemiotactic influence on regenerating axons originate from the target tissue (the nervous tissue) and are not modified by blood circulation. It is still unclear whether vascularization of the distal nervous end-organ and/or its connection to the periphery (intact nerve vs nerve graft) influences the maturation of nerve regeneration or not.27
In view of these unclear points, we believe that the pathways that will carry and join the proximal end to the targeted distal end should generate the ideal microenvironment in a transected nerve. To date, many studies have been designed to gain data on tissue specificity. Hence, the experiments did not allow investigation of other levels of specifity, such as end-organ and topographic.39 The reach of the axons to the distal nerve segments is not sufficient for ideal innervations. They must also reach their original end organ. Otherwise, the distal neurotubules would not be spread by original axons, and this is not a functional result. The neuron makes efforts to regain its original axoplasm volume by extending peripheral processes: the axons regenerate toward the periphery. An ongoing exchange of chemical messages between the center and the periphery by anterograde and retrograde axonal transport is essential for this process. Neurite-promoting factors promote growth cone formation and stimulate the advancement of axons by offering optimal and specific local molecular signals. Advancing growth cones are dependent on the presence of neurite-outgrowth-promoting factors in vivo as well as in vitro. In the extracellular matrix and in the basal laminae, which surround Schwann cells, the glycoproteins laminin and fibronectin may provide such support of axonal growth.2,40
In our study, proximal nerve axons that regenerated inside the horseshoe shaped nerve graft had the possibility to choose to grow towards either target nerve or to another distal target, or both. In neurotropism studies, while investigating the affinity rates of regenerating axons towards various tissues and the attractive power of various tissues for regenerating axons, the shortcomings of the experimental models should not mislead us. Most of the classic neurotropism studies have used either synthetic or non-neural tissues as a medium and guide. Many authors have mentioned the negative aspects of these environments. In our study, we aimed to eliminate this problem by basing our study design on the fact that a nerve can accomplish the best regeneration only within a nervous tissue. Reverse end-to-side nerve repair was first described by Isaachs and colleagues. I had published the results of my previous experimental study on the functional efficacy of reverse end-to-side repair.41 In the light of these findings, we know that this repair technique allows axonal regeneration from the main nerve trunk to both ends of the nerve. Thus, by this study, we were able to show that this repair model could be used in studies focusing on tissue neurotropism, and it would help us to eliminate the misleadings associated with the microenvironment.
As seen by the histomorphometric analysis, axons were observed to sprout towards both ends of the horseshoe-shaped nerve graft in a free fashion, without being affected by the negative influences of the microenvironment. If the regenerating nerve axons, either myelinated or non-myelinated, failed to reach the peroneal end organ, they were found to sprout mostly towards the tibial side. Furthermore, in Group 4, where there was no end organ, the number of nerves, both myelinated and non-myelinated, regenerating towards the tibial side, was determined to reach maximum values. These findings were verified statistically as well. As another significant data, as proposed in our experimental study, more myelinated axons are expected to be formed in Group 2 peroneal leg of our model compared to the myelinated nerves in the peroneal leg of Group 3. In the statistical comparison, as shown by the Mann Whitney U test analyzing the difference between myelinated axon counts in Group 2 and Group 3 on peroneal side (p:0.011), the mean myelinated axon count was higher in Group 2 (Table 2).
The most important limiting factor of this method is the requirement of an exact match between the diameter of the anastomotic area of reverse end-to-side nerve repair and the main nerve trunk diameter, along with the need for a very careful coaptation procedure. Otherwise, many theoretical advantages of this model may not be achieved. A suture reaction or a severe scar formation in the coaptation area may also shadow the success of the model.
In short, we know that a nerve regenerates best within a nervous tissue, and microenvironment acts as a very important factor throughout this process. It is also known that there are many factors that affect nerve regeneration. Therefore, we believe that reverse end-to-side nerve repair can be applied at least for eliminating other influential factors. By using this method, we may be able to measure the axonal affinity of various tissues in an exact way, establish a neurotropism scale for different tissues and thus form a new basis for the future nerve repair studies.
None.
None.
Author declares that there is no conflict of interest.
- 1. Thoenen H, Bandtlow C, Heumann R, et al. Nerve growth factor: cellular localization and regulation of synthesis. Cell Mol Neurobiol. 1988;8:35–40.
- 2. Lundborg G, Dahlin LB, Danielsen N, et al. Trophism, tropism, and specificity in nerve regeneration. J Reconstr Microsurg. 1994;10:345–354.
- 3. Tsubokawa N, Maki Y, Yoshizu T, et al. Comparison of the neurotropic effects of motor and sensory Schwann cells during regeneration of peripheral nerves. Scand J Plast Reconstr Hand Surg. 1999;33:379–385.
- 4. Lee JM, Tos P, Raimondo S, et al. Lack of topographic specificity in nerve fiber regeneration of rat forelimb mixed nerves. Neuroscience. 2007;144(3):985–990.
- 5. Forsman J. Zur Kenntniss der Neurotropismus. Weiter Beitrage. Beitrage zur pathologische Anatomie; 1990:27,407.
- 6. Cajal S, Ramon Y. Degeneration and Regeneration in the Nervous System. London: Oxford University Press; 1928.
- 7. Weiss P, Taylor AC. Further experimental evidence against 'neurotropism' in nerve regeneration. Journal of Experimental Zoology. 1994;95:233–257.
- 8. Weiss P. Experiments on cell and axon orientation in vitro: the role of colloidal exudates in tissue organization. Journal of Experimental Zoology. 1945;100:353–386.
- 9. Politis MJ, Ederle K, Spencer PS. Do Schwann cells guide regenerating axons by chemotaxis? Transactions of the American Society for Neurochemistry. 1981:12,247.
- 10. Politis MJ, Ederle K, Spencer PS. Tropism in nevre regeneration in vivo. Attraction of regenerating axons by diffusible factors derived from cells in distal nerve stumps of transected peripheral nerves. Brain Research. 1982;253:1–12.
- 11. Anderson PN, Turmaine M. Axonal regeneration through arterial grafts. Journal of Anatomy. 1986;147:73–82.
- 12. Lundborg G, Hansson HA. Nerve lesions with interruption of continuity: studies on the growth pattern of regenerating axons in the gap between the proximal and distal nerve ends. In: Posttraumatic Nerve Regeneration: Experimental Basis and Clinical Implications. Gorio A, Millesi H, Mingrino S editors. New York: Raven Press; 1981:229–239.
- 13. Ochii M. Experimental study on orientation of regenerating fibers in severed peripheral nerve. Hiroshima Journal of Medical Sciences. 1983;31:389–406.
- 14. MacKinnon S, Dellon L, Lundborg G, et al. A study of neurotropism in the primate model. Journal of Hand Surgery. 1986;11A:888–894.
- 15. Nachemson A, Lundborg G. A study of neurotropism in a primate model. Journal of Hand Surgery. 1986 11A, Proceedings,766.
- 16. Forssman J. Zur kenntniss der neurotropismus. [On the knowledge of neurotropism. Further results.] Weiter beitra¨ge. Beitr Pathol Anat. 1900;27:407–442.
- 17. Lundborg G, Longo FM, Varon S. Nerve regeneration model and trophic factors in vivo. Brain Res. 1982;232:157–161.
- 18. Lundborg G, editor. Nerve injury and repair. Edinburgh: Churchill Livingstone; 1988: 16-21.
- 19. Knoobs B, Hurtado H, Van Den Bosch De Aquilar P. Rat sciatic nerve regeneration within an acrylic semipermeable tube and comparison with a silicone impermeable material. Journal of Neuropathology and Experimental Neurology. 1990;49:438–448.
- 20. Glasby MA, Davies AH, Gattuso JM, et al. The effect of distal influences on peripheral nerve regeneration through muscle grafts. Neuro-Orthopedics. 1988;6:61–66.
- 21. Abernethy DA, Rud A, Thomas PK. Neurotropic influence of the distal stump of transected peripheral nerve on axonal regeneration: absence of topographic specificity in adult nerve. J Anat. 1992;180:395–400.
- 22. Frey M, Koller R, Liegl C, et al. Role of muscle target organ on the regeneration of motor nerve fibres in long nevre grafts: a synopsis of experimental and clinical data. Microsurgery. 1996;17:80–88.
- 23. Lundborg G, Dahlin LB, Danielsen N, et al. Tissue specificity in nerve regeneration. Scand J Plast Reconstr Surg. 1986;20:279–283.
- 24. Varon S, Adler R. Tropic and specifying factors directed to neuronal cells. Adv Cell Neurobiol. 1981;2:115–163.
- 25. Brushart TM, Seiler WA. Selective reinnervation of distal motor stumps by peripheral motor axons. Experimental Neurology. 1987;97:289–300.
- 26. Williams LR. Rat aorta isografts possess nerve regeneration promoting properties in silicon Y chambers. Experimental Neurology. 1987;97:555–563.
- 27. Tos P, Battiston B, Geuna S, et al. Tissue Specificity in Rat Peripheral Nerve Regeneration Through Combined Skaletal Muscle And Vein Conduit Grafts. Microsurgery. 2000;20:65–71.
- 28. Hudson AR, Hunter D, Kline DG, et al. Histological studies of experimental interfascicular graft repair. J Neurosurg. 1979;51:333–340.
- 29. Millesi H. Peripheral nerve injuries. Nerve sutures and nevre grafting. Scand J Plast Reconstr Surg. 1982;19:25–37.
- 30. Terzis JK, Sun DD, Thanos PK. Historical and basic science review: past, present and future of nerve repair. J Reconstr Microsurg. 1997;13:215–225.
- 31. Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312–318.
- 32. Brooke R, Seckel MD. Enhancement Peripheral Nerve Regeneration. Muscle and Nerve. 1990;13:785–800.
- 33. Lundborg G, Rosen B, Dahlin L, et al. Tubular repair of the median or ulnar nerve in the human forearm: A 5-year follow-up. J Hand Surg [Br]. 2004;29:100–107.
- 34. David S, Aguayo AJ. Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science. 1981;214:931–933.
- 35. Tomita K, Kubo T, Matsuda K, et al. Effect of Conduit Repair on Aberant Motor Axon Growth Within The Nerve Graft In Rats. Microsurgery. 2007;27:500–509.
- 36. Battiston B, Geuna S, Ferrero M, et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nevre repair. Microsurgery. 2005;25:258–267.
- 37. Geuna S, Papalia I, Tos P. End-to-side (terminolateral) nevre regeneration: A challenge for neuroscientists coming from an intriguing nerve repair concept. Brain Res Rev. 2006;52:381–388.
- 38. Lundborg G. Nerve injury and repair. Edinburgh: Churchill Livingstone; 2005.
- 39. Evans PJ, Bain JR, Mackinnon SE, et al. Selective reinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559:315–321.
- 40. Ülkü E, Yüksel F, Açıkel C, et al. Comparison of Functional Results of Nerve Graft, Vein Graft, and Vein Filled With Muscle Graft in End to Side Neurorraphy. Microsurgery. 2003;3:40–48.
- 41. Balik Ozan, Menderes Adnan. A Successful Neurotization of Two Different Muscles Using a Single Intact Motor Nerve: Experimental Study on Rats. Annals of Plastic Surgery. 2011;66(2):172–178.

