Traditional medicine has received more attention in recent years. Numerous plants have been employed in medicine to treat neurological disorders like AD and other memory-related problems. Traditional uses for dietary, spice, and food additive medicinal purposes included the use of, Nigella sativa, Crocus sativus, Ferula assafoetida, Coriandrum sativum, Zataria multiflora, Thymus vulgaris Cats Claw, and Carotenoids, monoterpenes, and polyphenol chemicals, which are the main ingredients in these plants, improved neurological processes. These healing plants improved antioxidant production, reduced oxidative stress, and prevented the neurological system's acetylcholinesterase enzyme from working. Reduced production of proinflammatory cytokines such IL-6, TNF-an ,IL-1b, NF-Kβ, Bax Protein, Bcl-2,Caspase-3 and total nitrite is another way that plants are neuroprotective. As a result, the effects of the aforementioned medications and their active ingredients improved neurodegenerative diseases, indicating their therapeutic promise in diseases like AD and depression that are linked to neuro-inflammation and neurotransmitter deficit.
Keywords: Medicinal spice herbal plant, Memory nervous system, Traditional medicine
Parkinson's disease (PD), Alzheimer's disease (AD) and (MS) are examples of neurodegenerative diseases that cause delayed neuronal death along with loss of cognitive and sensory abilities.1 These diseases have recently been linked to several multifactorial etiologies, societal issues, and financial difficulties.2,3 Anti-inflammatory medications may also halt the progression of neurodegenerative illnesses like AD. (NSAIDs) may lower the chance of developing Alzheimer's disease , according to numerous research.4,5 In Parkinson's disease (PD), neuronal degeneration is caused by pathological processes such as inflammation, oxidative stress, apoptosis, mitochondrial malfunction, and hereditary factors.6 In AD7 and PD, it has been suggested that increased lipid peroxidation may kill off dopaminergic and cholinergic neurons.8 The brain contains a variety of enzyme-based and non-enzymatic antioxidants, including total thiol groups superoxide dismutase (SOD).9,10 The (CNS) is especially susceptible to peroxidation processes since it also contains a high level of PFA.9 The brain has less antioxidant activity than other tissues, which makes it more vulnerable to oxidative damage.11 Plant body part like leaves, roots, stems, fruits, seeds and flowers were employed supplementary therapies in traditional medicine, curcumin, Resveratrol, polyphenols, ginsenoside, triptolide, and other herbal extracts have neuroprotective properties.12 phytochemicals found in herbal products. The herb's component(s) has (have) more biological activity.13,14
The aim of the present evaluation study was to emphasize the beneficial effects of numerous medicinal plants that have historically been used for dietary, spice, food additive, and diverse therapeutic purposes on induced neurotoxicity. 3
Up until the end of August 2023, the information for this review was gathered from databases like, Web of Science, PubMed, Scopus and Google Scholar. The terms "neuroprotective" or "neurotoxicity" were also used, as well as "Crocus sativus," "Nigella sativa," "Coriandrum sativum," "Ferula assafoetida," "Thymus vulgaris," "Zataria multiflora," and "Curcuma longa." Other search terms included "Ginkgo biloba" " All research that resulted in alterations in neurotransmitter release, behavioral changes, oxidant/antioxidant pro-inflammatory cytokines and parameters, were included, including in vitro studies, review articles, animal studies and clinical trials. The exclusion criteria included unpublished data and letters to the editor.
Crocus sativus
Saffron, is a member of the Crocoideae superfamily and is grown in a number of nations, with Afghanistan, Iran, and Spain, Turkey.15 A small piece of the yellowish style from the C. sativus plant is linked to the dried, dark-red stigma that makes up saffron. In many parts of the world, it is primarily utilized as an herbal medication.16 There are 150 distinct chemicals in saffron, including sugars, polypeptides, lipids, water, minerals, and vitamins. The primary biologically active components of saffron are crocins, a group of carotenoids that are all red, water-soluble, Picrocrocin, another saffron component with a bitter flavor.17
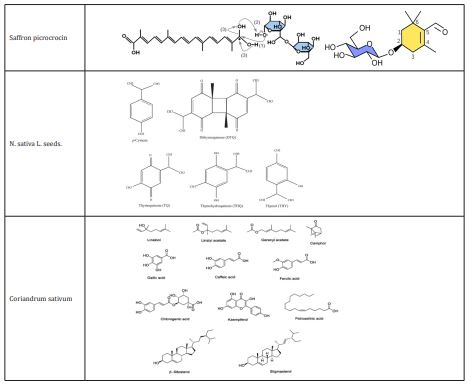
C. sativus of medicinal properties
Crocus sativus is a plant used in traditional Iranian medicine to address mental health issues. Components of C. sativus have recently been utilized to relax smooth muscle and treat various neurological diseases.18e20 C. sativus extract has been shown to have anticonvulsant and anti-Alzheimer effects in both human and animal models.18-20 Crocin, the primary component of C. sativus, has powerful antioxidant effects by lowering MDA levels.21,22 In addition, mice with aluminum chloride-induced neurotoxicity were treated for 45 days with Zaffron extract (200mg/kg) and honey syrup.23,24 The effectiveness of Zaffron (30mg/day) for the treatment of mild-to-moderate AD in patients 55 years and older was found to be on par with donepezil, and the frequency of side effects from saffron extract, with the exception of vomiting, was on par with donepezil.25 Similar to this, 46 patients with mild-to-moderate Alzheimer disease experienced enhanced cognitive functioning after using C.Sative for 16 weeks.26 Similar to the effects of fluoxetine and imipramine (100mg/day), C.Sative (30mg/day) was effective in the treatment of depression for 6 weeks.27 The findings demonstrated that C. sativus 80 mg and fluoxetine (30mg/day) and were more beneficial than C. sativus 40mg for treating mild to moderate depressive disorders.28
Nigella sativa
The annual plant Nigella sativa L., often known as Nigella sativa, is a member of the Ranunculaceae family and is commonly grown in the Mediterranean region as well as in, the Middle East, Western Asia and Eastern Europe. Various Persian delicacies, including bread, pickles, sauces, and salads, have been spiced with Nigella sativa seeds.29 Oil, protein, glucose, and fiber are among the chemical components of Nigella sativa seeds.oleic acid, Linoleic acid, arachidic acid, palmitic acid, stearic acid, eicosadienoic acid, myristic acid linoleic acid are the chemical components of N. sativa's fixed oil.30 P-cymene (37.3%), Thymoquinone (TQ) (13.7%), Carvacrol (11.77%), and Thymol (0.33%) are the main phenolic chemicals found in N. sativa seeds.29-32
Medicinal properties of N. sativa
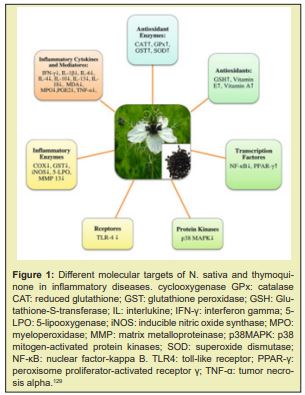
The medicinal plant N. sativa is well known for having strong antioxidant properties.N. sativa is said to have preventive benefits against kidney injury.33,34 The cognitive deficiencies in spatial awareness brought on by chronic cerebral hypo perfusion in rats could be greatly improved by N. sativa seeds.35 Furthermore, N. sativa lowered the AChE activity and oxidative stress in the rats' brains and restored learning and memory deficits brought on by scopolamine.36 N. sativa oil's antioxidant effects on rheumatoid arthritis (RA) patients revealed that N. sativa decreased the levels of IL-10, MDA, and NO in the blood. Additionally, N. sativa lowered oxidative stress and enhanced inflammatory responses in RA patients.37 Neuroprotective effects of thymoquinone (TQ) and N. sativa on various nervous system disorders such as epilepsy AD, and neurotoxicity have been reviewed Figure 1.38-41
Coriandrum sativum
The herb coriander which belongs to the Apiaceae family of parsleys, is an annual. In Persian, this plant is typically referred to as Geshniz. The Mediterranean region is the plant's natural habitat, and Coriandrum sativum is widely cultivated worldwide.42,43 Fresh herb oil is primarily composed of aliphatic aldehydes (primarily C10-C16 aldehydes), which have a fetid scent,44 while the oil extracted from coriander fruit is primarily composed of linalool and a few other oxygenated monoterpenes and monoterpene hydrocarbons.45 Additionally, coriander contains a significant number of essential oils (EO), which are crucial for brain and development activities, as well as lipids like petroselinic acid. Linalool, linoleic, and linolenic acids make up most of the coriander essential oil.46 Linalool (60–70%) and hydrocarbons (20%) are present in coriander seed oil, although the herb oil's makeup is entirely different from that of the seed oil.47
Medicinal properties of C. sativum
The antibacterial and antirheumatic properties of C. sativum seed extract make it a popular ingredient in lotions and shampoos.48 C. sativum has been advocated for use in Iranian traditional medicine to treat sleeplessness.49,50 Before going to bed, it has been recommended to take a single dose of crushed plant seeds, fresh leaf extract, and tea to help with anxiety and sleeplessness.49 Other traditional treatments have been found utilize C. sativum seed in similar ways.51 The anxiolytic effect of the C. sativum leaves extract (200mg/kg) was demonstrated by an increase in the amount of time spent in open arms and the proportion of open arm entrances.52 Using the maximal electroshock seizure paradigm and pentylenetetrazole (PTZ) as an anticonvulsant, the anticonvulsant activity of coriander seed aqueous (0.5g/kg, i.p.) and ethenolic (3.5 and 5g/kg, i.p.) extracts was investigated. 53,54
Ferula assafoetida
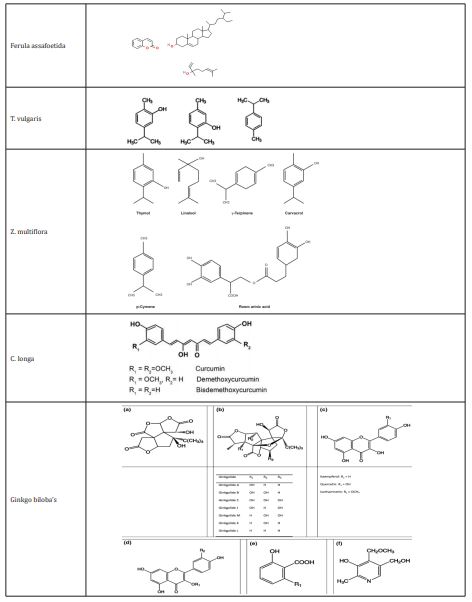
which is derived from the exudates of the plant's living subterranean tap roots, rhizome is a member of the Apiaceae family. In Iran, F. assafoetida, also known as gum-resin, is called "Anghouzeh," "Khorakoma," and "Anguzakoma."55 25 chemicals were found in the hydro distilled oil, with e-1-propyl sec-butyl disulfide being a significant component.56
F. assafoetida of medicinal properties
Researchers are interested in F. asafoetida (Apiaceae) because of its therapeutic and dietary benefits. Plants' roots, new shoots, and leaves are consumed as vegetables. The root of the plant, Ferula asafoetida, is used as an antipyretic, and its leaves are anthelmintic, carminative, and diaphoretic.57 epilepsy, Asthma, , flatulence, stomachaches, sluggish digestion, intestinal parasites and influenza are only a few of the conditions that are treated with F. asafoetida in traditional medicine.58 Also mentioned are the sedative, analgesic, expectorant , stimulant, antiperiodic, carminative, antipasmodic, ant-diabetic , vermifuge, emmenagogue, anti-inflammatory, laxative, contraceptive, and anti-epileptic actions of F. asafoetida oleo-gum resin.59 Studies have been done on F. asafoetida's effects on muscarinic receptors and potential pathways for functional antagonistic effects on guinea-pig tracheal smooth muscle.60,61 On smooth muscles, F. assafoetida has a relaxing effect. Its potential processes have been explored.62 Acute and sub chronic toxicity of F. asafoetida was assessed in a study, and the findings showed that neither a single oral treatment of this plant (500 mg/kg) nor repeated doses (250mg/kg) over the course of 28 days did not result in rat mortality or evident toxicological indications.63 The oleo gum resin of F. asafoetida has also been shown to improve regeneration and re-myelination while reducing lymphocyte infiltration in neuropathic tissue in mice.64 Additionally, research has indicated that F. asafoetida resin may inhibit monoamine oxidase B which makes it a viable treatment for neurological diseases including and Alzheimer’s and Parkinson's.65 Meanwhile, acetylcholinesterase (AChE) inhibition by Ferula asafoetida has been demonstrated in vivo as well as in vitro on the neurological system of the snail. Researchers have hypothesized that Ferula asafoetida's ability to improve memory may be due to its ability to inhibit AChE in the rat brain.66 Rats' memories were enhanced by the plant extract in dose-dependent ways in behavioral models including raised plus maze. The higher dose (400mg) of the extract improved memory in the passive avoidance test, whereas the lesser amount (200mg) had no effect. 67
Thymus vulgaris
There are about 38 species of this plant, which is found throughout subtropical regions.68 The primary ingredients in TV are phenols, carvacrol (15%) and thymol (40%). In the winter, it has lower phenol concentrations. The essential oil also contains cineol, cymen, pinene, borneol, thymol methyl ether (2%), and esters.68
Medicinal properties of T. vulgaris
Thyme is a common ingredient in herbal teas and infusions in traditional medicine.69 Bioactive thyme components have been shown to have antioxidant, antibacterial, antitussive, antispasmodic, and expectorant properties. Examples of these compounds include thyme essential oil (TEO) constituents, natural terpenoid thymol, flavonoids and phenolic acids and phenol isomer carvacrol.70,71 Tocols and phenolic in thymus vulgaris oil (TO) have been found to directly interact with free radicals and reduce lipid peroxidation, according to research.72 Additionally, it has been found that thymol therapy improves the antioxidant state in rat brain tissue.73 Additionally, the results of behavioral experiments have shown that when given orally to rats for a week, thyme extract can have anxiolytic effects. In support of this claim, thyme extract increases the proportion of entry and the amount of time spent in the maze's welcoming arms.74 The elevated plus maze (EPM) in mice exhibits anxiolytic effects due to kaempferol in thyme extract, according to the findings of animal experiments.75 It has also been proposed that the thyme essential oil has a dose-dependent protective effect against the toxicity of alfatoxins.76-78 Thymol also regulates the GABAA receptor and operates centrally by simulating or enhancing GABA function, according to research.79 Thymol, a bioactive monoterpene derived from the thymus, has recently been shown to have neuroprotective and ameliorative effects on cognitive impairment induced on by scopolamine or amyloid b in rats.80 Researchers have hypothesized that thymol's possible impact on GABA-mediated regulation of synaptic transmission may explain its neuroprotective properties.81 Researchers discovered that TO could improve nicotinic Ach receptor activity, synaptic acetylcholine (Ach) which in turn could modify cholinergic function.82
Zataria multiflora
The Lamiaceae family includes Zataria multiflora (also known as Z. multiflora).83 multiflrol (1), Multi-flotriol (2), a novel aromatic ester of p-hydroxy benzoic acid (3), and 3 recognized constituents— luteolin, dihydroxyaromadendrane , and a-tocopherolquinone—are all p-cymene derivatives.84-86 carvacrol (33.65%), Thymol (37.59%), PARA-cymene g-terpinene (3.88%), (7.72%), and b-caryophyllene (2.06%) made up the majority of the plant oil.87
Z. multiflora of medicinal properties
luteolin, Terpens, di-, tri-,6-hydroxyluteolin glycosides and tetraethoxylated chemicals, among others, are present in Z. multiflora and may contribute to its medicinal effects.88 Multifloral Z Boiss essential oil (ZEO) has preservation properties, but its strong flavor and scent have prevented it from being used in large quantities as a food preservative.89 The plant's analgesic, antibacterial, and digestive properties are employed in traditional Iranian medicine.88 Additionally, it has been shown that Z. multiflora essential oil exhibits in vitro antibacterial, antioxidant, and antifungal activities.89,90 According to research, pomegranate juice wasn't able to match the ZEO's powerful antioxidative effects.89 This plant has also been linked to anti-inflammatory, immune-regulatory, and anti-bacterial activities. Additionally, it has been claimed that administering Z. multiflora essential oil intravenously to rats could reverse the learning and memory deficits produced by Ab. Researchers therefore believed that zataria multiflora essential oil was a valuable source of a natural medicinal agent for reducing the cognitive symptoms of (AD).91-93
Curcuma longa
Southeast Asian nations cultivate the Zingiberaceae family plant known as curcuma longa (C. longa).94 The flavonoid curcumin (diferuloylmethane), along with a number of volatile oils like atlantone, tumerone, and zingiberone, are what make turmeric active. Sugars, proteins, and resins are additional ingredients. The most thoroughly studied active component, curcumin, makes up 0.3% to 5.4% of raw turmeric.95
C. longa of medicinal properties
Curcumin is a naturally occurring polyphenol and non-flavonoid substance found in some plants, including Curcuma longa. Kulkarni stated that dopamine, norepinephrine, and 5-HT levels in the CNS can be increased by curcumin water soluble extract.96 Inhibitory effects of curcumin derived from Curcuma longa have been seen in cell culture and animal models for PD, apoptosis, ROS production, cytokines production, platelet aggregation, cognitive impairments and brain oxidative damage.97,98 On oxidative 99 and renal damage, 1000mg/kg of C. longa extract taken orally has been shown to be protective.100 Curcumin (50, 100, or 200mg/kg) has reportedly been administered to rats to treat cognitive impairments and indications of mitochondrial dysfunction.101 Additionally, there is evidence that curcumin has neuroprotective benefits in cerebral ischemia and neuronal degenerative diseases. The rat brain is protected against focal ischemia by curcumin thanks to the increase of the transcription factors Nrf2 and HO-1 expression. 102-104 Additionally, researchers hypothesized that curcumin inhibits ER stress-related TXNIP/NLRP3 inflammatory activation, which is thought to exacerbate glutamate neurotoxicity in the rat hippocampus.105-107 Curcumin's antioxidant capabilities are also connected to its neuroprotective benefits in PD. Wang observed that 6-OHDA-exposed human cell line SH-SY5Y showed curcumin restored ROS intracellular accumulation.108,109 Curcumin (60mg/kg, body weight, orally) treatment for three weeks reduced the degeneration of striatum neurons in rats with 6-OHDA damage.110 By increasing the GSH levels, curcumin shielded the neurons against ROS.Curcumin enhanced SOD levels in the 6-OHDA-lesioned striatum of mice111 and 6-OHDA was produced in MES23.5 cells112.The axons have been said to be protected from LPS degeneration by curcumin.113 The inducible nitric oxide synthase (iNOS) antagonist BCl-2 may be overexpressed, which would then mediate the neuroprotective effects of curcumin. Curcumin is therefore useful in reducing NO-mediated degeneration. Curcumin also reduced NF-kB activation in LPS.114-118 The plant's interaction with the GABA and opioid system may have analgesic and anticonvulsant properties. Plants on various disorders as clinical studies were shown in various mechanisms of therapeutic characteristics of medicinal herbs were summarized in Table 1.
Medicinal plant of ginkgo biloba’s
The eastern Chinese province of Zhejiang is thought to be the origins of ginkgo biloba.129,130 (AD) and other forms of dementia are treated with standardized (GBE), which is made from the dried leaves of the ginkgo tree.131, 132 The cognitive function of the aged and Alzheimer's disease patients was improved in a number of clinical investigations,133–137 however GBE in AD is not supported by other studies. After the publishing of two significant trials, the debate about the advantages of ginkgo biloba for various purposes has been stronger. 1) DeKosky and colleagues' GEM research138 i) The study by McCarney and colleagues139 Notably, numerous changes in energy metabolism, stabilization of the mitochondrial membrane potential, inhibition of cytochrome c release, upregulation of the anti-apoptotic Bcl-2 protein and downregulation of the pro-apoptotic Bax protein, reduced levels of caspase 3 and caspase 9 after oxidative stress, and a decrease in apoptotic cell death were reported. These changes on the apoptotic pathway and mitochondrial function appear to be crucial for their beneficial effects.140-146
Origin and history of ginkgo biloba
The world's oldest living tree species is the ginkgo biloba. The earliest known occurrence of the Ginkgo species was during the Permian Period, between 286 and 248 million years ago. The only remaining member of the Ginkgo family is Ginkgo biloba. Buddhist monks who cared for the trees on holy sites are credited with their cultivation and preservation, as well as the trees' unusual malleability and disease resistance.147 Frank Lloyd Wright loved gingko, which quickly made its way into American cityscapes.148 Ginkgo has been used medicinally for approximately 5000 years, mostly in the treatment of asthma, in China.149
Leaf extract of ginkgo biloba
Ginkgo leaves have been utilized for therapeutic purposes for a very long time. Early in the 1970s, Karlsruhe, Germany's Dr. Ginkgo biloba extract preparation and created highly concentrated and stable extracts from Ginkgo biloba leaves.150 According to some theories, the pharmacologically active components of Ginkgo biloba are flavonoids and terpenoids.151,152 Ginkgo biloba 's solubility in water is caused by the presence of organic acid.153 The significant investigations of Y Luo and others that demonstrate the neuroprotective properties of this herbal extract in cell and animal-based models have engaged in a variety of preclinical study assessing Ginkgo biloba effects.154–160 With varying degrees of success, Ginkgo biloba has been used to treat and prevent neurodegenerative dementias brought on by aging, Parkinson's disease (PD), Alzheimer's disease (AD), and neurosensory disorders (such as tinnitus).161,162 Ginkgo biloba to treat multi-infarct dementia, depression, cerebral insufficiency, stroke, thrombosis, myocardial ischemia and (POAD), which is characterized by symptoms like memory loss, difficulty concentrating, anxiety, and confusion. Additionally, its effects on traumatic brain damage, hypertension, and sexual dysfunction brought on by antidepressants163 have been investigated.164
Components of GBE
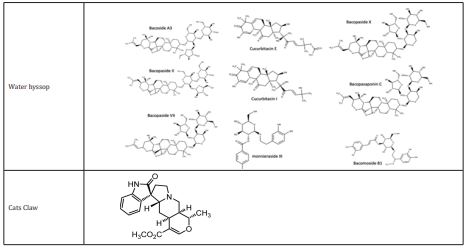
Ginkgo biloba is made up of a variety of components, including trilactonicditerpenes, ginkgolides A, B, and C (ginkgolides J and M are also present, but at a lower level) Figure 1. There are also flavonoids such, proanthocyanidins isorhamnetins, trilactonic sesquiterpene and quercetin. Other components have also been identified, including, ginkgolic acid, D-glucaric acid, hydroxykynurenic, rhamnose, protocatechuic, kynurenic, shikimic acids and vanillic. In particular, it has been demonstrated that ginkgolides operate as (PAF) antagonists, preventing platelet aggregation and promoting blood flow.165 Due to their phenolic structures, flavonoids are recognized to be among the most potent antioxidants among different polyphenols and to also function as heavy metal chelators.166 They have been studied clinically in cardiovascular problems167 Additionally, in preclinical models of AD168 and stroke, Ginkgo biloba demonstrated neuroprotective and anti-inflammatory characteristics.169
Pharmacological importance
Despite existing for more than 200 million years, ginkgo has only recently received significant research attention. Due to its incredible vibrancy, there has been an increase in research into its potential use in food, supplements, and health. Fresh or dried leaves and seeds are the main components of the ginkgo tree that have medicinal benefits. It contains a lot of active substances that are recognized to have pharmacological value Figure 2. The active ingredient in GBE derived from ginkgo leaves enhances blood circulation, strengthens capillary walls, prevents clot formation, and guards against damage to nerve cells from oxygen deprivation. The leaf extracts are used to treat dementia diseases like memory loss and focus issues. The extract also has anti-asthmatic properties.170 Anti-oxidant,171 anti-inflammatory,172 anti-radical173 and neuroprotective capabilities against neurodegenerative diseases as AD174, 175 and PD.176 While complex mixes like blueberries or chocolate offer benefits due to the preventing AD. Pure antioxidants may also interfere with tightly controlled stress responses. AD, memory improvement, dementia of vascular origin, cognitive abnormalities, and positive benefits of the Ginkgo biloba plant's antioxidative actions in combination with other medications that increase their efficacy Figure 3.177,178 In cases of brain damage, ginkgo biloba can act as a neuroprotective medication and a reinforcing antidepressant.179 This plant alters cerebral blood flow, which may lessen weariness and inattentiveness.180 Other investigations181,182 GBE has beneficial effects on schizophrenia, depression, anxiety, and psychosis.184 It also promotes cerebral blood circulation.
Neurotherapeutic Effects of GBE Extract
Numerous in vitro and in vivo model studies have supported the neuroprotective effect of GBE.185 Studies carried out in vitro shown that GBE shielded cultured neurons from degeneration brought on by hydrogen peroxide, hypoxia verapamil, amyloid, glutamate186,199 nitric oxide (NO) 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP. focal cerebral ischemia in mice and rats, hypoxia heat stress, sub chronic cold stress,200-208 and amphetamine-induced behavioral sensitization have all been shown to reduce neuronal damage when administered intravenously or orally with EGb761209 and in an amyotrophic lateral sclerosis transgenic mice model.210 In addition to its ability to scavenge free radicals, EGb 761 has also been demonstrated to influence the transcription of various genes involved in the control of oxidative stress.211 This is a crucial characteristic of EGB 761 since it may promote cellular tolerance to oxidative stress, safeguarding neuronal cells from oxidative damage frequently linked to neurological disorders like AD and PD. 212-221
Water hyssop medicinal plant
Bacopa monnieri (L.) Wettst., is a creeping, bitter-tasting plant that grows in wet, marshy areas.222 We predicted that this plant extract could lessen memory loss and neurodegeneration in animal models of Alzheimer's disease based on its reputation as a nerve tonic and its antioxidant activities. Astragalus, Brahmi,223-225 medicine, Bacopa monnieri is used to cure a variety of diseases and boost memory.217 Bacopa monnieri may enhance cognition, particularly memory and learning, according to systematic evaluations of early research,227-228 however the effect wasn't noticeable until after many weeks of treatment.226 2019 saw the FDA send letters of warning to producers of dietary supplements containing Bacopa monnieri for making unsubstantiated and unlawful health claims about the treatment or prevention of Alzheimer's disease, gastrointestinal disease, hypoglycemia, anxiety and blood pressure. The FDA claimed that none of these or any other medicinal uses for Bacopa monnieri products have been authorized.223-226
Cats claw medicinal plant
Furthermore, the interaction of its antioxidant activity with free radicals superoxide anion (O2), 1, 1-diphenyl-2-picrylhydrazyl (DPPH), and hydroxyl (HO), peroxy (ROO), and hypochlorous acid (HOCl) and hydrogen peroxide (H2O2), has been assessed.227-229 Uncaria tomentosa extract contains quinic acid (QA), a biologically active substance that has the ability to suppress NF-KB expression. If QA contains antioxidant action is unknown. Batch-2 is a brand-new aqueous cat's claw extract. In this study, we examine batch-2's antioxidative activity and protective effect on 6-OHDA-induced apoptosis in SH-SY5Y cells using QA as an internal reference.230 Studies in cell cultures are the only source of evidence for anti-inflammatory activity. And has not been proven in randomized control human trials.231-232 Numerous other conditions, such as HIV, Crohn's disease, multiple sclerosis, systemic lupus erythematosus (SLE or lupus), endometriosis, kidney issues, bladder cancer, and Alzheimer's disease are being examined in relation to cat's claw. Before scientists can determine whether it is beneficial, more study is required.233
The effects of the aforementioned medications and their active ingredients enhanced neurological diseases, indicating their therapeutic promise in diseases like AD and depression that are linked to neuro-inflammation and neurotransmitter deficit.
Iriti234 reported that the focus will be on the and anti-inflammatory activity and antioxidant exhibited by particular molecules found in or remedies food plants. recommended by herbal medicines, as these are important factors in the etiopathogenesis of both neurodegenerative and neurological disorders.
Faridzadeh235 studied Aromatic plants with antioxidant, anti-inflammatory, and neuroprotective properties include, lavender (Lavandula angustifolia), sage (Salvia officinalis) and rosemary (Salvia Rosmarinus). Additionally, they have demonstrated promise in the treatment of common neurological conditions such as AD, PD,migraines, and cognitive impairments.
Ratheesh236 investigated Natural remedies and medicinal plants, including wolfberry, ginseng, curcumin, resveratrol, Baccopa monnieri and Withania somnifera (ashwagandha), GBE have been used to treat neurological disorders and symptoms that have been documented in in vivo or clinical trials. These medicinal plants against Neurodegenerative diseases.
Roy237 one of the information collected With an emphasis on their mode of action and therapeutic potential, this review will emphasize the significance of herbal plants and their phytoconstituents against neurodegenerative disorders and other related conditions.
da Costa238 suggested that the Using the herbs and essential oils of many species of medicinal plants is one way to combat this, since they include a number of bioactive components and phytochemicals that have the ability to protect neurons. Furthermore, they have favorable responses to neurological conditions such dementia, oxidative stress, anxiety, cerebral ischemia, and oxidative toxicity, indicating their potential utility as supplemental therapeutic agents for the management of neurological conditions.
Khan239 studied Our aim was to succinctly illustrate the benefits of various berries in terms of our comprehension of the pathogenetic pathways underlying neurodevelopmental disorders and neurodegenerative diseases.
Khan240 recommended drug the 6-Amino Flavone is an effective neurotherapeutic drug in Neurological disorders causing neurodegeneration.
Khan241 reported that Flavonoids is a neuroprotective agent against Neurogenerative diseases.
We suggest concentrating on neurotoxicity in various research (in vivo and in vitro) and examining the effects of therapeutic herbs on the brain system in this review. The aforementioned medicinal plants function in protective ways by raising SOD and catalase levels, restoring GSH, lowering MDA levels, and protecting neurons from free radical damage through antioxidant activities. These natural substances may have some protective effects due to a decrease in Ca2+, Na+, and an increase in K+ levels, or a "anti-glutamatergic" action. The aforementioned plants have neuroprotective properties that reduce inflammatory cytokines while increasing anti-inflammatory cytokines, inhibit acetylcholinesterase activity, reduce MDA levels in the nervous system by modulating glutamatergic and GABAergic neurons, and increase amino acids and serotonin (5-HT) levels in the neurotransmitter systems. Additionally, scientific and clinical studies showed that several herbs have anti-inflammatory, antioxidant, and immunoregulatory effects on a variety of diseases. This discovery assisted in recommending the usage of these herbs and key natural resource compounds.
None.
None.
The Authors declare that there are no conflicts of interest.
- Mattson MP. Metal-catalyzed disruption of membrane protein and lipid signaling in the pathogenesis of neurodegenerative disorders. Ann N Y Acad Sci. 2004;1012:37-50.
- Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71(1):35-48.
- Breitner JC. The role of anti-inflammatory drugs in the prevention and treatment of Alzheimer's disease. Annu Rev Med. 1996;47:401-411.
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425-432.
- Fu W, Zhuang W, Zhou S, Wang X. Plant-derived neuroprotective agents in Parkinson's disease. Am J Transl Res. 2015;7(7):1189-1202.
- Olcese JM, Cao C, Mori T, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. 2009;47(1):82-96.
- Khaldy H, Escames G, León J, et al. Comparative effects of melatonin, L-deprenyl, Trolox and ascorbate in the suppression of hydroxyl radical formation during dopamine autoxidation in vitro. J Pineal Res. 2000;29(2):100-107.
- Camello Almaraz C, Gomez Pinilla PJ, Pozo MJ, et al. Age‐related alterations in Ca2+ signals and mitochondrial membrane potential in exocrine cells are prevented by melatonin. Journal of pineal research. 2008;45(2):191-198.
- Tewari A, Mahendru V, Sinha A, et al. Antioxidants: The new frontier for translational research in cerebroprotection. J Anaesthesiol Clin Pharmacol. 2014;30(2):160-171.
- Floyd RA, Carney JM. Free radical damage to protein and DNA: mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32 Suppl:S22-S27.
- Virmani A, Pinto L, Binienda Z, et al. Food, nutrigenomics, and neurodegeneration--neuroprotection by what you eat!. Mol Neurobiol. 2013;48(2):353-362.
- Suk K. Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases. A focus on traditional medicines and flavonoids. Neurosignals. 2005;14(1-2):23-33.
- Adams M, Gmünder F, Hamburger M. Plants traditionally used in age related brain disorders--a survey of ethnobotanical literature. J Ethnopharmacol. 2007;113(3):363-381.
- Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4(2):83-86.
- Jalali Heravi M, Parastar H, Ebrahimi Najafabadi H. Characterization of volatile components of Iranian saffron using factorial-based response surface modeling of ultrasonic extraction combined with gas chromatography-mass spectrometry analysis. J Chromatogr A. 2009 ;1216(33):6088-6097.
- Bathaie SZ, Mousavi SZ. New applications and mechanisms of action of saffron and its important ingredients. Crit Rev Food Sci Nutr. 2010;50(8):761-786.
- Khazdair MR, Boskabady MH, Hosseini M, et al. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J Phytomed. 2015;5(5):376-391.
- Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline. 2007;2:367-370.
- Mokhtari Zaer A, Khazdair MR, Boskabady MH. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J Phytomed. 2015;5(5):365-375.
- Karimi E, Oskoueian E, Hendra R, et al. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15(9):6244-6256.
- Tamaddonfard E, Farshid AA, Ahmadian E, et al. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran J Basic Med Sci. 2013;16(1):83-90.
- Saleem S, Ahmad M, Ahmad AS, et al. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J Med Food. 2006;9(2):246-253.
- Shati AA, Elsaid FG, Hafez EE. Biochemical and molecular aspects of aluminium chloride-induced neurotoxicity in mice and the protective role of Crocus sativus L. extraction and honey syrup. Neuroscience. 2011;175:66-74.
- Akhondzadeh S, Sabet MS, Harirchian MH, et al. Saffron in the treatment of patients with mild to moderate Alzheimer's disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35(5):581-588.
- Akhondzadeh S, Shafiee Sabet M, Harirchian MH, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer's disease. Psychopharmacology (Berl). 2010;207(4):637-643.
- Noorbala AA, Akhondzadeh S, Tahmacebi Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97(2):281-284.
- Moosavi SM, Ahmadi M, Amini M, et al. The effects of 40 and 80 mg hydro-alcoholic extract of Crocus sativus in the treatment of mild to moderate depression. Journal of mazandaran university of medical sciences. 2014;24(113):48-53.
- Hajhashemi V, Ghannadi A, Jafarabadi H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother Res. 2004;18(3):195-199.
- El Tahir K E D H, Bakeet DM. The black seed Nigella sativa Linnaeus-A mine for multi cures: a plea for urgent clinical evaluation of its volatile oil. Journal of Taibah University Medical Sciences. 2006;1(1):1-19.
- Tiruppur Venkatachallam SK, Pattekhan H, Divakar S, et al. Chemical composition of Nigella sativa L. seed extracts obtained by supercritical carbon dioxide. J Food Sci Technol. 2010;47(6):598-605.
- Kacem R, Meraihi Z. Effects of essential oil extracted from Nigella sativa (L.) seeds and its main components on human neutrophil elastase activity. Yakugaku Zasshi. 2006;126(4):301-305.
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323-328.
- Mohebbati R, Abbsnezhad A, Khajavi Rad A, et al. Effect of hydroalcholic extract of Nigella sativa on doxorubicin-induced functional damage of kidney in rats. Internal Medicine Today, 2016;22(1):3-20.
- Azzubaidi MS, Saxena AK, Talib NA, Ahmed QU, et al. Protective effect of treatment with black cumin oil on spatial cognitive functions of rats that suffered global cerebrovascular hypoperfusion. Acta Neurobiol Exp (Wars). 2012;72(2):154-165.
- Hosseini M, Mohammadpour T, Karami R, et al. Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin J Integr Med. 2015;21(6):438-444.
- Hadi V, Kheirouri S, Alizadeh M, et al. Effects of Nigella sativa oil extract on inflammatory cytokine response and oxidative stress status in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled clinical trial. Avicenna J Phytomed. 2016;6(1):34-43.
- Bin Sayeed MS, Asaduzzaman M, Morshed H, et al. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148(3):780-786.
- Bin Sayeed MS, Shams T, Fahim Hossain S, et al. Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. J Ethnopharmacol. 2014;152(1):156-162.
- Khazdair MR. The Protective Effects of Nigella sativa and Its Constituents on Induced Neurotoxicity. J Toxicol. 2015;2015:841823.
- Small E. Culinary Herbs. Ottawa: NRC Research Press. 1997.
- Lawrence BM. A planning scheme to evaluate new aromatic plants for the flavor and fragrance industries. In: Janick J, Simon JE, eds. New Crops. New York: Wiley. 1993:620e627.
- Potter TL. Essential oil composition of cilantro. J Agric Food Chem. 1996;44:1824e1826.
- Bandoni AL, Mizrahi I, Juárez MA. Composition and quality of the essential oil of coriander (Coriandrum sativum L.) from Argentina. Journal of essential oil research, 1998;10(5):581-584.
- Sahib NG, Anwar F, Gilani AH, et al. Coriander (Coriandrum sativum L.): a potential source of high-value components for functional foods and nutraceuticals--a review. Phytother Res. 2013;27(10):1439-1456.
- Guenther E. The Essential Oil. Florida, USA: REK Publishing Company. 1950.
- Yusuf M, Chowdhuty JU, Wahab MA, Begum J Medicinal Plants of Bangladesh. Bangladesh: Bangladesh Council of Scientific and Industrial Research; 1994.
- Mir Heidar H. Coriandrum sativum. Applic Plants Prev Treat Illnesses. 1992;1:247-252.
- Zargari A. Medicinal Plants. Zargari A. Medicinal Plants. 1991.
- Pathan AR, Kothawade KA, Logade MN. Anxiolytic and analgesic effect of seeds of Coriandrum sativum Linn. Int J Res Pharm Chem. 2011;1(4):1087-1099.
- Mahendra P, Bisht S. Anti-anxiety activity of Coriandrum sativum assessed using different experimental anxiety models. Indian J Pharmacol. 2011 Sep;43(5):574-577.
- Hosseinzadeh H, Madanifard M. Anticonvulsant effects of Coriandrum sativum L. seed extracts in mice. Archives of Iranian Medicine. 2000;3(4):1-4.
- Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J Ethnopharmacol. 2011;134(1):1-10.
- Khajeh M, Yamini Y, Bahramifar N, et al. Comparison of essential oils compositions of Ferula assa-foetida obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food chemistry. 2005;91(4):639-644.
- Zia-Ul-Haq M, Shahid SA, Ahmad S, et al. Antioxidant potential of various parts of Ferula assafoetida L. Journal of Medicinal Plants Research. 2012;6(16):3254-3258.
- Lee CL, Chiang LC, Cheng LH, et al. Influenza A (H(1)N(1)) Antiviral and Cytotoxic Agents from Ferula assa-foetida. J Nat Prod. 200;72(9):1568-1572.
- Bagheri SM, Rezvani ME, Vahidi AR, et al. Anticonvulsant effect of Ferula assa-foetida oleo gum resin on chemical and amygdala-kindled rats. N Am J Med Sci. 2014;6:408.
- Khazdair MR, Boskabady MH, Kiyanmehr M, et al. effects of Ferula assafoetida on muscarinic receptors of Guinea-pig tracheal smooth muscle. Jundishapur Journal of Natural Pharmaceutical Products. 2015;10(3).
- Kiyanmehr M, Boskabady MH, Khazdair MR, Hashemzehi M. Possible Mechanisms for Functional Antagonistic Effect of Ferula assafoetida on Muscarinic Receptors in Tracheal Smooth Muscle. Malays J Med Sci. 2016;23(1):35-43.
- Khazdair MR, Boskabady MH. The relaxant effect of Ferula assafoetida on smooth muscles and the possible mechanisms. Journal of HerbMed Pharmacology, 2015;4(2):40-44.
- Goudah A, Abdo-El-Sooud K, Yousef MA. Acute and subchronic toxicity assessment model of Ferula assa-foetida gum in rodents. Vet World. 2015;8(5):584-589.
- Homayouni Moghadam F, Dehghan M, Zarepur E, et al. Oleo gum resin of Ferula assa-foetida L. ameliorates peripheral neuropathy in mice. J Ethnopharmacol. 2014;154(1):183-189.
- Zarmouh NO, Messeha SS, Elshami FM, et al. Natural Products Screening for the Identification of Selective Monoamine Oxidase B Inhibitors. European J Med Plants. 2016;15(1):14802.
- Kumar P, Singh VK, Singh DK. Kinetics of enzyme inhibition by active molluscicidal agents ferulic acid, umbelliferone, eugenol and limonene in the nervous tissue of snail Lymnaea acuminata. Phytother Res. 2009;23(2):172-177.
- Vijayalakshmi, Adiga S, Bhat P, et al. Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44(1):82-87.
- Azaz AD, Irtem HA, Kurkcuoğlu M, et al. Composition and the in vitro antimicrobial activities of the essential oils of some Thymus species. Z Naturforsch C J Biosci. 2004;59(1-2):75-80.
- Lee SJ, Umano K, Shibamoto T, et al. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food chemistry, 91(1):131-137.
- Dogu Baykut E, Gunes G, Decker EA. Impact of shortwave ultraviolet (UV-C) radiation on the antioxidant activity of thyme (Thymus vulgaris L.). Food Chem. 2014;157:167-173.
- Farina M, Franco JL, Ribas CM, et al. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol. 2005;57(11):1503-1508.
- Ramadan MF. Cold pressed thyme (Thymus vulgaris) oil. Cold Pressed Oils, 2020;719-724.
- Youdim KA, Deans SG. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br J Nutr. 2000;83(1):87-93.
- Komaki A, Hoseini F, Shahidi S, et al. Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J Tradit Complement Med. 2015;6(3):257-261.
- Grundmann O, Nakajima J, Kamata K, et al. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine. 2009;16(4):295-302.
- Melo FH, Venâncio ET, de Sousa DP, et al. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: involvement with GABAergic transmission. Fundam Clin Pharmacol. 2010;24(4):437-443.
- Souto Maior FN, de Carvalho FL, de Morais LC, et al. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol Biochem Behav. 2011;100(2):259-263.
- El Nekeety AA, Mohamed SR, Hathout AS, et al. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon. 2011;57(7-8):984-991.
- Waliwitiya R, Belton P, Nicholson RA, et al. Effects of the essential oil constituent thymol and other neuroactive chemicals on flight motor activity and wing beat frequency in the blowfly Phaenicia sericata. Pest Manag Sci. 2010;66(3):277-289.
- Deng XY, Li HY, Chen JJ, et al. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res. 2015;291:12-19.
- Marín LD, Sánchez Borzone M, García DA. Comparative antioxidant properties of some GABAergic phenols and related compounds, determined for homogeneous and membrane systems. Med Chem. 2011;7(4):317-324.
- Sammi SR, Trivedi S, Rath SK, et al. 1-Methyl-4-propan-2-ylbenzene from Thymus vulgaris Attenuates Cholinergic Dysfunction. Mol Neurobiol. 2017;54(7):5468-5481.
- Ali MS, Saleem M, Ali Z, et al. Chemistry of zataria multiflora (lamiaceae). Phytochemistry, 2000;55(8):933-936.
- Bohlmann F, Grenz M, Jakupovic J, et al. Four heliangolides and other sesquiterpenes from Brasilia sickii. Phytochemistry, 1983;22(5):1213-1218.
- Sashida Y, Nakata H, Shimomura H, et al. Sesquiterpene lactones from pyrethrum flowers. Phytochemistry. 1983;22(5):1219-1222.
- Rasool N, Khan AQ, Ahmad VU, et al. A benzoquinone and a coumestan from Psoralea plicata. Phytochemistry, 1991;30(8):2800-2803.
- Sharififar F, Moshafi MH, Mansouri SH, et al. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food control. 2007;18(7):800-805.
- Boskabady MH, Gholami Mhtaj L. Effect of the Zataria multiflora on systemic inflammation of experimental animals model of COPD. Biomed Res Int. 2014;2014:802189.
- Bazargani Gilani B, Tajik H, Aliakbarlu J. Physicochemical and antioxidative characteristics of Iranian pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) juice and comparison of its antioxidative activity with Zataria multiflora Boiss essential oil. Vet Res Forum. 2014;5(4):313-318.
- Dadashi M, Hashemi A, Eslami G, et al. Evaluation of antibacterial effects of Zataria multiflora Boiss extracts against ESBL-producing Klebsiella pneumoniae strains. Avicenna J Phytomed. 2016;6(3):336-343.
- Shokri H, Asadi F, Bahonar AR, et al. The Role of Zataria multiflora Essence (Iranian herb) on Innate Immunity of Animal Model. Iran J Immunol. 2006;3(4):164-168.
- Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000;73(3):379-385.
- Majlessi N, Choopani S, Kamalinejad M, et al. Amelioration of amyloid β-induced cognitive deficits by Zataria multiflora Boiss. essential oil in a rat model of Alzheimer's disease. CNS Neurosci Ther. 2012;18(4):295-301.
- Araújo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96(5):723-728.
- Akram M, Shahab Uddin AA, Usmanghani KHAN, et al. Curcuma longa and curcumin: a review article. Rom J Biol Plant Biol. 2010;55(2):65-70.
- Kulkarni SK, Akula KK, Deshpande J. Evaluation of antidepressant-like activity of novel water-soluble curcumin formulations and St. John's wort in behavioral paradigms of despair. Pharmacology. 2012;89(1-2):83-90.
- Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892-5901.
- Yang J, Song S, Li J, et al. Neuroprotective effect of curcumin on hippocampal injury in 6-OHDA-induced Parkinson's disease rat. Pathol Res Pract. 2014;210(6):357-362.
- Khazdair MR, Mohebbati R, Karimi S, et al. The protective effects of Curcuma longa extract on oxidative stress markers in the liver induced by Adriamycin in rat. Physiology and Pharmacology, 2016;20(1):31-37.
- Mohebbati R, Shafei MN, Soukhtanloo M, et al. Adriamycin-induced oxidative stress is prevented by mixed hydro-alcoholic extract of Nigella sativa and Curcuma longa in rat kidney. Avicenna J Phytomed. 2016;6(1):86-94.
- Khatri DK, Juvekar AR. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2016;150-151:39-47.
- Liu L, Zhang W, Wang L, et al. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res. 2014;39(7):1322-1331.
- Tu XK, Yang WZ, Chen JP, et al. Curcumin inhibits TLR2/4-NF-κB signaling pathway and attenuates brain damage in permanent focal cerebral ischemia in rats. Inflammation. 2014;37(5):1544-1551.
- Yang C, Zhang X, Fan H, et al. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133-141.
- Li Y, Li J, Li S, et al. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol Appl Pharmacol. 2015;286(1):53-63.
- Li L, Li H, Li M. Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med. 2015;8(9):14985-14991.
- Li X, Zhao L, Yue L, et al. Evidence for the protective effects of curcumin against oxyhemoglobin-induced injury in rat cortical neurons. Brain Res Bull. 2016;120:34-40.
- Wang J, Du XX, Jiang H, et al. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem Pharmacol. 2009;78(2):178-183.
- Jaisin Y, Thampithak A, Meesarapee B, et al. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci Lett. 2011;489(3):192-196.
- Agrawal SS, Gullaiya S, Dubey V, et al. Neurodegenerative Shielding by Curcumin and Its Derivatives on Brain Lesions Induced by 6-OHDA Model of Parkinson's Disease in Albino Wistar Rats. Cardiovasc Psychiatry Neurol. 2012;2012:942981.
- Harish G, Venkateshappa C, Mythri RB, et al. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: Implications for Parkinson's disease. Bioorg Med Chem. 2010;18(7):2631-2638.
- Tripanichkul W, Jaroensuppaperch EO. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci. 2013;17(10):1360-1368.
- Tegenge MA, Rajbhandari L, Shrestha S, et al. Curcumin protects axons from degeneration in the setting of local neuroinflammation. Exp Neurol. 2014;253:102-110.
- Chen J, Tang XQ, Zhi JL, et al. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11(6):943-53.
- Ojha RP, Rastogi M, Devi BP, et al. Neuroprotective effect of curcuminoids against inflammation-mediated dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. J Neuroimmune Pharmacol. 2012;7(3):609-618.
- Yang S, Zhang D, Yang Z, et al. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem Res. 2008;33(10):2044-2053.
- Kianmehr M, Rezaei A, Hosseini M, et al. Immunomodulatory effect of characterized extract of Zataria multiflora on Th1, Th2 and Th17 in normal and Th2 polarization state. Food Chem Toxicol. 2017;99:119-127.
- Khazdair MR, Ghorani V, Alavinezhad A, et al. Pharmacological effects of Zataria multiflora Boiss L. and its constituents focus on their anti-inflammatory, antioxidant, and immunomodulatory effects. Fundam Clin Pharmacol. 2018;32(1):26-50.
- Akhondzadeh S, Fallah Pour H, Afkham K, et al. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial. BMC Complement Altern Med. 2004;4:12.
- Akhondzadeh S, Tahmacebi Pour N, Noorbala AA, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19(2):148-151.
- Moshiri E, Basti AA, Noorbala AA, et al. Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13(9-10):607-611.
- Moosavi SM, Ahmadi M, Amini M, et al. The effects of 40 and 80 mg hydro-alcoholic extract of Crocus sativus in the treatment of mild to moderate depression. J Mazandaran Univ Med Sci (JMUMS). 2014;24.
- Boskabady MH, Javan H, Sajady M, et al. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21(5):559-566.
- Boskabady MH, Farhadi J. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14(9):1137-1144.
- Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17(10):707-713.
- Kalus U, Pruss A, Bystron J, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209-1214.
- Waheed A, Miana GA, Ahmad SI. Clinical investigation of hypoglycemic effect of seeds of Azadirachta-inidca in type-2 (NIDDM) diabetes mellitus. Pak J Pharm Sci. 2006;19(4):322-325.
- Prucksunand C, Indrasukhsri B, Leethochawalit M, et al. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health. 2001;32(1):208-215.
- Amin B, Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta medica, 2015;82:8-16.
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819-828.
- Singh SK, Sinha P, Mishra L, et al. Neuroprotective Role of a Novel Copper Chelator against Aβ 42 Induced Neurotoxicity. Int J Alzheimers Dis. 2013;2013:567128.
- Kumar A, Srivastava S, Tripathi S, et al. Molecular insight into amyloid oligomer destabilizing mechanism of flavonoid derivative 2-(4' benzyloxyphenyl)-3-hydroxy-chromen-4-one through docking and molecular dynamics simulations. J Biomol Struct Dyn. 2016;34(6):1252-1263.
- Singh SK, Gaur R, Kumar A, et al. The flavonoid derivative 2-(4' Benzyloxyphenyl)-3-hydroxy-chromen-4-one protects against Aβ42-induced neurodegeneration in transgenic Drosophila: insights from in silico and in vivo studies. Neurotox Res. 2014;26(4):331-350.
- Klafki HW, Staufenbiel M, Kornhuber J, et al. Therapeutic approaches to Alzheimer's disease. Brain. 2006;129(Pt 11):2840-2855.
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311-335.
- Asadi S, Ahmadiani A, Esmaeili MA, et al. In vitro antioxidant activities and an investigation of neuroprotection by six Salvia species from Iran: a comparative study. Food Chem Toxicol. 2010;48(5):1341-1349.
- Singh SK, Srivastav S, Yadav AK, et al. Overview of Alzheimer's Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds. Oxid Med Cell Longev. 2016;2016:7361613.
- Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51(5):1014-1026.
- Kubitzki K. In: Kramer K, Greech PS, editors. The families and genera of vascular plants— pteridophytes and gymnosperms. Berlin: Springer Verlag. 1990.p.284.
- Hori T, Ridge RW, Tulecke W, et al. Ginkgo biloba a global treasure: from biology to medicine Springer Science & Business Media. 2012.
- Vellas B, Coley N, Ousset PJ, et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer's disease (GuidAge): a randomised placebo-controlled trial. Lancet Neurol. 2012;11(10):851-859.
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55(11):1409-1415.
- Kanowski S, Herrmann WM, Stephan K, et al. Proof of efficacy of the ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry. 1996;29(2):47-56.
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278(16):1327-1332.
- Napryeyenko O, Borzenko I; GINDEM-NP Study Group. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57(1):4-11.
- Yancheva S, Ihl R, Nikolova G, et al. Ginkgo biloba extract EGb 761(R), donepezil or both combined in the treatment of Alzheimer's disease with neuropsychiatric features: a randomised, double-blind, exploratory trial. Aging Ment Health. 2009;13(2):183-190.
- Mix JA, Crews WD Jr. A double-blind, placebo-controlled, randomized trial of Ginkgo biloba extract EGb 761 in a sample of cognitively intact older adults: neuropsychological findings. Hum Psychopharmacol. 2002;17(6):267-277.
- DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo Evaluation of Memory (GEM) Study Investigators. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253-2262.
- McCarney R, Fisher P, Iliffe S, et al. Ginkgo biloba for mild to moderate dementia in a community setting: a pragmatic, randomised, parallel-group, double-blind, placebo-controlled trial. Int J Geriatr Psychiatry. 2008;23(12):1222–1230.
- Schindowski K, Leutner S, Kressmann S, et al. Age-related increase of oxidative stress-induced apoptosis in mice prevention by Ginkgo biloba extract (EGb761). J Neural Transm (Vienna). 2001;108(8-9):969-978.
- Smith JV, Burdick AJ, Golik P, et al. Anti-apoptotic properties of Ginkgo biloba extract EGb 761 in differentiated PC12 cells. Cell Mol Biol (Noisy-le-grand). 2002;48(6):699-707.
- Leuner K, Hauptmann S, Abdel Kader R, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease?. Antioxid Redox Signal. 2007;9(10):1659-1675.
- Abdel Kader R, Hauptmann S, Keil U, et al. Stabilization of mitochondrial function by Ginkgo biloba extract (EGb 761). Pharmacol Res. 2007;56(6):493-502.
- Luo Y, Smith JV, Paramasivam V, et al. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci U S A. 2002;99(19):12197-12202.
- Eckert A, Keil U, Scherping I, et al. Stabilization of mitochondrial membrane potential and improvement of neuronal energy metabolism by Ginkgo biloba extract EGb 761. Ann N Y Acad Sci. 2005;1056:474-485.
- Eckert A, Keil U, Kressmann S, et al. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36:S15-S23.
- Kwant, Cor. Hiroshima: A Bombed Ginkgo. The Ginkgo.
- Wanadoo. http://perso.wanadoo.fr/ginkgo.dm/index.html. Internet Explorer April 18, 2006.
- Griggs B. GREEN PHARMACY: The History and Evolution of Western Herbal Medicine. New York: Viking Press. 1981:p.326.
- Le Bars PL. Magnitude of effect and special approach to Ginkgo biloba extract EGb 761 in cognitive disorders. Pharmacopsychiatry. 2003;36:S44-S49.
- Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J Ethnopharmacol. 1996;50(3):131-139.
- Shi C, Zhao L, Zhu B, et al. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem Biol Interact. 2009;181(1):115-123.
- Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67(3):235-257.
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60(9):1779-1792.
- Christen Y. Ginkgo biloba and neurodegenerative disorders. Front Biosci. 2004;9:3091-3104.
- Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64(4):465-472.
- Luo Y. Ginkgo biloba neuroprotection: Therapeutic implications in Alzheimer's disease. J Alzheimers Dis. 2001;3(4):401-407.
- Saleem S, Zhuang H, Biswal S, et al. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39(12):3389-3396.
- Ponto LL, Schultz SK. Ginkgo biloba extract: review of CNS effects. Ann Clin Psychiatry. 2003;15(2):109-119.
- Luo Y. Alzheimer's disease, the nematode Caenorhabditis elegans, and ginkgo biloba leaf extract. Life Sci. 2006;78(18):2066-2072.
- Holstein N. Ginkgo-Spezialextrakt EGb 761 in der Tinnitus-Therapie. Eine Ubersicht über die Ergebnisse der durchgeführten klinischen Prüfungen [Ginkgo special extract EGb 761 in tinnitus therapy. An overview of results of completed clinical trials]. Fortschr Med Orig. 2001;118(4):157-164.172.
- Schneider B. Ginkgo-biloba-Extrakt bei peripheren arteriellen Verschlusskrankheiten. Meta-Analyse von kontrollierten klinischen Studien [Ginkgo biloba extract in peripheral arterial diseases. Meta-analysis of controlled clinical studies]. Arzneimittelforschung. 1992;42(4):428-436.
- Cohen AJ, Bartlik B. Ginkgo biloba for antidepressant-induced sexual dysfunction. J Sex Marital Ther. 1998;24(2):139-143.
- Diamond BJ, Shiflett SC, Feiwel N, et al. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81(5):668-678.
- Yue TL, Feuerstein GZ. Platelet-activating factor: a putative neuromodulator and mediator in the pathophysiology of brain injury. Crit Rev Neurobiol. 1994;8(1-2):11-24.
- Gohil K, Moy RK, Farzin S, et al. mRNA expression profile of a human cancer cell line in response to Ginkgo biloba extract: induction of antioxidant response and the Golgi system. Free Radic Res. 2000;33(6):831-849.
- Dower JI, Geleijnse JM, Gijsbers L, et al. Supplementation of the Pure Flavonoids Epicatechin and Quercetin Affects Some Biomarkers of Endothelial Dysfunction and Inflammation in (Pre)Hypertensive Adults: A Randomized Double-Blind, Placebo-Controlled, Crossover Trial. J Nutr. 2015;145(7):1459-1463.
- Dower JI, Geleijnse JM, Gijsbers L, et al. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: a randomized, double-blind, placebo-controlled, crossover trial. Am J Clin Nutr. 2015;101(5):914-921.
- Yin Y, Ren Y, Wu W, et al. Protective effects of bilobalide on Aβ(25-35) induced learning and memory impairments in male rats. Pharmacol Biochem Behav. 2013;106:77-84.
- Jiang M, Li J, Peng Q, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 2014;11:167.
- Mahmoud F, Abul H, Onadeko B, et al. In vitro effects of Ginkgolide B on lymphocyte activation in atopic asthma: comparison with cyclosporin A. Jpn J Pharmacol. 2000;83(3):241-245.
- Nakanishi K. Terpene trilactones from Gingko biloba: from ancient times to the 21st century. Bioorg Med Chem. 2005;13(17):4987-5000.
- Bairy KL. Wound healing potentials of plant products. J Nat Rem. 2002;2(1):11–20.
- Louajri A, Harraga S, Godot V, et al. The effect of ginkgo biloba extract on free radical production in hypoxic rats. Biol Pharm Bull. 2001;24(6):710-712.
- Zeng K, Li M, Hu J, et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr Alzheimer Res. 2018;15(1):89-99.
- Yuan Q, Wang CW, Shi J, et al. Effects of Ginkgo biloba on dementia: An overview of systematic reviews. J Ethnopharmacol. 2017;195:1-9.
- Srivastav S, Fatima M, Mondal AC. Important medicinal herbs in Parkinson's disease pharmacotherapy. Biomed Pharmacother. 2017;92:856-863.
- Smith TC, Ryan MA, Smith B, et al. Complementary and alternative medicine use among US Navy and Marine Corps personnel. BMC Complement Altern Med. 2007;7:16.
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr Drug Targets. 2000;1(1):25-58.
- Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry. 2006;188:109-121.
- Hechtman L. Attention –deficit / Hyperactivity disorder. In: Sadock BJ, Sadock VA, eds. Comprehensive Text Book of Psychiatry. 8th edn. Philadelphia: Lippincott Williams & Wilkins;2005.
- Noorbala AA, Akhondzadeh S. Attention-deficit/hyperactivity disorder: etiology and pharmacotherapy. Arch Iran Med. 2006;9(4):374-380.
- Himi T, Saito H, Nishiyama N. Effect of ginseng saponins on the survival of cerebral cortex neurons in cell cultures. Chem Pharm Bull (Tokyo). 1989;37(2):481-484.
- Garg RK, Nag D, Agrawal A. A double blind placebo controlled trial of ginkgo biloba extract in acute cerebral ischaemia. J Assoc Physicians India. 1995;43(11):760-763.
- White HL, Scates PW, Cooper BR. Extracts of Ginkgo biloba leaves inhibit monoamine oxidase. Life Sci. 1996;58(16):1315-1321.
- Singh SK, Barreto GE, Aliev G, et al. Ginkgo biloba as an Alternative Medicine in the Treatment of Anxiety in Dementia and other Psychiatric Disorders. Curr Drug Metab. 2017;18(2):112-119.
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60(9):1779-1792.
- Ni Y, Zhao B, Hou J, et al. Preventive effect of Ginkgo biloba extract on apoptosis in rat cerebellar neuronal cells induced by hydroxyl radicals. Neurosci Lett. 1996;214(2-3):115-118.
- Oyama Y, Chikahisa L, Ueha T, et al. Ginkgo biloba extract protects brain neurons against oxidative stress induced by hydrogen peroxide. Brain Res. 1996;712(2):349-352.
- Chen C, Wei T, Gao Z, et al. Different effects of the constituents of EGb761 on apoptosis in rat cerebellar granule cells induced by hydroxyl radicals. Biochem Mol Biol Int. 1999;47(3):397-405.
- Song W, Guan HJ, Zhu XZ, et al. Protective effect of bilobalide against nitric oxide-induced neurotoxicity in PC12 cells. Acta Pharmacol Sin. 2000;21(5):415-420.
- Xin W, Wei T, Chen C, et al. Mechanisms of apoptosis in rat cerebellar granule cells induced by hydroxyl radicals and the effects of EGb761 and its constituents. Toxicology. 2000;148(2-3):103-110.
- Zhou LJ, Zhu XZ. Reactive oxygen species-induced apoptosis in PC12 cells and protective effect of bilobalide. J Pharmacol Exp Ther. 2000;293(3):982-988.
- Guidetti C, Paracchini S, Lucchini S, et al. Prevention of neuronal cell damage induced by oxidative stress in-vitro: effect of different Ginkgo biloba extracts. J Pharm Pharmacol. 2001;53(3):387-392.
- Klein J, Chatterjee SS, Löffelholz K. Phospholipid breakdown and choline release under hypoxic conditions: inhibition by bilobalide, a constituent of Ginkgo biloba. Brain Res. 1997;755(2):347-350.
- Krieglstein J, Ausmeier F, El Abhar H, et al. Neuroprotective effects of Ginkgo biloba constituents. Eur J Pharm Sci. 1995;3(1):39–48.
- Zhu L, Gao J, Wang Y, et al. Neuron degeneration induced by verapamil and attenuated by EGb761. J Basic Clin Physiol Pharmacol. 1997;8(4):301-314.
- Bastianetto S, Ramassamy C, Doré S, et al. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. Eur J Neurosci. 2000;12(6):1882-1890.
- Zhou LJ, Song W, Zhu XZ, et al. Protective effects of bilobalide on amyloid beta-peptide 25-35-induced PC12 cell cytotoxicity. Acta Pharmacol Sin. 2000;21(1):75-79.
- Yang SF, Wu Q, Sun AS, et al. Protective effect and mechanism of Ginkgo biloba leaf extracts for Parkinson disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Acta Pharmacol Sin. 2001;22(12):1089-1093.
- Bastianetto S, Zheng WH, Quirion R. The Ginkgo biloba extract (EGb 761) protects and rescues hippocampal cells against nitric oxide-induced toxicity: involvement of its flavonoid constituents and protein kinase C. J Neurochem. 2000;74(6):2268-2277.
- Zhang WR, Hayashi T, Kitagawa H, et al. Protective effect of ginkgo extract on rat brain with transient middle cerebral artery occlusion. Neurol Res. 2000;22(5):517-521.
- Rabin O, Drieu K, Grange E, et al. Effects of EGb 761 on fatty acid reincorporation during reperfusion following ischemia in the brain of the awake gerbil. Mol Chem Neuropathol. 1998;34(1):79-101.
- Calapai G, Crupi A, Firenzuoli F, et al. Neuroprotective effects of Ginkgo biloba extract in brain ischemia are mediated by inhibition of nitric oxide synthesis. Life Sci. 2000;67(22):2673-2683.
- Chandrasekaran K, Mehrabian Z, Spinnewyn B, et al. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36:S89-S94.
- Oberpichler H, Beck T, Abdel Rahman MM, et al. Effects of Ginkgo biloba constituents related to protection against brain damage caused by hypoxia. Pharmacol Res Commun. 1988;20(5):349-368.
- Sharma HS, Drieu K, Alm P, et al. Role of nitric oxide in blood-brain barrier permeability, brain edema and cell damage following hyperthermic brain injury. An experimental study using EGB-761 and Gingkolide B pretreatment in the rat. Acta Neurochir Suppl. 2000;76:81-86.
- Bolaños Jiménez F, Manhães de Castro R, Sarhan H, et al. Stress-induced 5-HT1A receptor desensitization: protective effects of Ginkgo biloba extract (EGb 761). Fundam Clin Pharmacol. 1995;9(2):169-174.
- Trovero F, Brochet D, Tassin JP, et al. Ginkgo biloba extract EGb761 reduces the development of amphetamine-induced behavioral sensitization: effects on hippocampal type II corticosteroid receptors. Brain Res. 1999;818(1):135-139.
- Ferrante RJ, Klein AM, Dedeoglu A, et al. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J Mol Neurosci. 2001;17(1):89-96.
- Gohil K, Packer L. Global gene expression analysis identifies cell and tissue specific actions of Ginkgo biloba extract, EGb 761. Cell Mol Biol (Noisy-le-grand). 2002;48(6):625-631.
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi. 1956.
- Bhattacharya SK, Ghosal S. Anxiolytic activity of a standardized extract of Bacopa monniera: an experimental study. Phytomedicine. 1998;5(2):77-82.
- Bhattacharya SK, Bhattacharya A, Kumar A, e al. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res. 2000;14(3):174-179.
- William A Correll, Jr "FDA Warning Letter: Peak Nootropics LLC aka Advanced Nootropics". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. 2019.
- William A Correll, Jr. "FDA Warning Letter: TEK Naturals". Office of Compliance, Center for Food Safety and Applied Nutrition, Inspections, Compliance, Enforcement, and Criminal Investigations, US Food and Drug Administration. 2019.
- Mammone T, Akesson C, Gan D, et al. A water soluble extract from Uncaria tomentosa (Cat's Claw) is a potent enhancer of DNA repair in primary organ cultures of human skin. Phytother Res. 2006;20(3):178-183.
- Pilarski R, Zieliński H, Ciesiołka D, et al. Antioxidant activity of ethanolic and aqueous extracts of Uncaria tomentosa (Willd.) DC. J Ethnopharmacol. 2006;104(1-2):18-23.
- Zhen Hua SHI, Zhao Wilson XI, Hao WANG, et al. The Neuroprotective Effect of Batch 鄄 2, an Aqueous Extract From Cat 忆 s Claw (Uncaria tomentosa) on 6 鄄 OHDA 鄄 Induced SH 鄄 SY5Y Cell Damage. Progress in Biochemistry and Biophysics. 2010;37(7):769-778.
- Assessment report on Uncaria tomentosa (Willd. ex Schult.) DC., cortex. European Medicines Agency. 2015.
- Cat's claw. National Center for Complementary and Integrative Health, US National Institutes of Health. 2020.
- Sandoval Chacón M, Thompson JH, Zhang XJ, et al. Anti inflammatory actions of cat's claw: the role of NF-kappaB. Aliment Pharmacol Ther. 1998;12(12):1279-1289.
- Miller Mark JS, Zhang Xiao Jing, Charbonnet Randi M, et al. The Anti-Inflammatory Actions of the Herbal Medicine, Cat's Claw, Are Due to a Suppression of NF-κB Activation and Inhibition of Gene Expression. Pediatric Research. 1999;45(7):114.
- Gattuso M, Di Sapio O, Gattuso S, et al. Morphoanatomical studies of Uncaria tomentosa and Uncaria guianensis bark and leaves. Phytomedicine. 2004;11(2-3):213-223.
- Nowak A, Kojder K, Zielonka Brzezicka J, et al. The Use of Ginkgo Biloba L. as a Neuroprotective Agent in the Alzheimer's Disease. Front Pharmacol. 2021;12:775034.
- Eskandari Roozbahani N, Shomali T, Taherianfard M. Neuroprotective Effect of Zataria Multiflora Essential Oil on Rats With Alzheimer Disease: A Mechanistic Study. Basic Clin Neurosci. 2019;10(1):85-97.
- Iriti M, Vitalini S, Fico G, et al. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules. 2010;15(5):3517-3555.
- Faridzadeh A, Salimi Y, Ghasemirad H, et al. Neuroprotective Potential of Aromatic Herbs: Rosemary, Sage, and Lavender. Front Neurosci. 2022;16:909833.
- Ratheesh G, Tian L, Venugopal JR, et al. Role of medicinal plants in neurodegenerative diseases. Biomanufacturing Reviews. 2017;2:1-16.
- Roy S, Awasthi H. Herbal medicines as neuroprotective agent: A mechanistic approach. Int J Pharm Pharm Sci. 2017;9:1-7.
- da Costa IM, Pedrosa E C G A, de Carvalho Bezerra A P, et al. Extracts and Essential Oils from Medicinal Plants and Their Neuroprotective Effect. In Neuroprotection-New Approaches and Prospects. London, UK: IntechOpen.
- Khan WA, Khan A, Khan I, et al. Berry fruits advances in understanding the pathogenetic mechanisms of neurodevelopmental disorders and neurodegenerative diseases. Pure and Applied Biology (PAB). 2023;12(1):637-652.
- Khan WA, Shah S A, Khan S. The 6-AF Evaluation of Neuroprotective Activity against Cd- Induced Oxidative Stress and Degenerative Brain Disease including PD in Mice. Annals of Psychophysiology. 2022;9(2):76–84.
- Khan WA. Therapeutic Potential of Natural Pharmacological Agents: Flavonoids against Various Diseases. Scholastic: Journal of Natural and Medical Education. 2022;1:39-53.

