Heat Island effect was caused by the difference in the effects of the sun heating the urban and suburban region where there are abundant of constructions and plants respectively, and it brought some problems about the living environment for the people. The urban region where there is almost only concrete and iron made constructions became too hot and sometimes unacceptable for people to stay, and the haze is also hard to eliminate by afforestation, haze is significant a risk to people’s health. However, this effect could also be positively used. As the heat island causes temperature gradient between the city center and the suburban area where the afforestation is more abundant, the city could be redesigned as a structure of center-industry and surrounding-living; thereby, not only the region where people live would be cooler and healthier, but also the heat island would be utilized as a huge chimney to extract the industrial pollution including haze out of the city, making the suburban regions safer and afforestation is effective again to eliminating haze from where people live. A full understanding about entropy in a view of thermodynamics as well as its application to the photosynthesis are given, the photosynthesis in plants absorbed the heat from the sunlight and stored it in forms of chemical energy inside the carbohydrates produced throughout the synthesis generates minus entropy compared to the sunlight directly dissipated into heat in urban region, the energy from the sunlight as well as the minus entropy thereof, which is a potential for heat to dissipate, was stored in the products of photosynthesis, i.e., the carbohydrates etc. which provide food for the whole biosphere on the earth; therefore, the temperature would be significantly lowered by plants in afforested regions.
Keywords: Heat island, Pollution, Afforestation, Photosynthesis, Entropy, Thermodynamics1
Heat island effect is a noticeable problem in today’s Metropolises. It is an effect that the urban region is always much warmer than the surrounding suburban region where there are more afforested. As the Figure 1 shows, there is a major difference in regions from the center of a city to its suburban region.
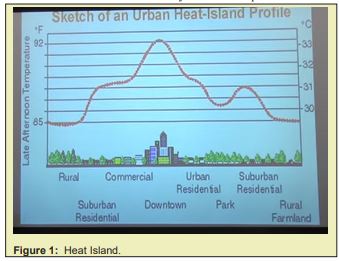
The heat island effect currently is thought to be caused by the difference in heat capacity between the afforested region and the urban region where there is less afforestation and more buildings made of concrete, and the afforested suburban region is also wetter, the evaporation of water would also cool down the region. However, there is also another important factor that wasn’t noticed but have significant contribution to the heat island effect: photosynthesis. And the measurement of cooling down the city by watering doesn’t seem to lower down the temperature of the region unless there is a fountain. And if it was caused by the difference in heat capacity, the afforested region would be warmer at night while the urban part where there is only dry concrete constructure would cool down, while factually this is not true. In region between the plants where there is no fountain, one could still feel those green creatures like a radiant refrigerator, it feels cool around it.
Photosynthesis is a process that absorbs light as its energy source and utilize it to produce food which saves the energy it absorbed as chemical energy in its products, carbohydrate is a major product of it, and it is the ultimate source of the food for creatures as well as the human on the earth. However, the photosynthesis seems to be something can solely convert the energy in photos which may dissipate into heat directly to be energy stored in the products of the synthesis, saying carbohydrates as the form of chemical energy, releasing oxygen, moreover any plants which are capable of photosynthesis can infinite reproduce by human’s scientific breeding, such as Professor Yuán Lóng Píng’s hybrid rice. Actually, the plants are using the minus entropy stored from their seeds to solely convert the energy in photons to be chemical energy in their products, the energy cold be used to do works. Counting man’s cultivation to breed more productive seeds, the creation of more photosynthesis is a sole process that converts heat into work, and there is only man’s laboring with no other effect as ruled by the second law of thermodynamics. The second law of thermodynamics assumes that there is no sole process that can convert the heat into work or from a low temperature reservoir to one of a higher temperature, while the sole effect of photosynthesis seems to be totally reverse to it. Is the second law wrong? It used to be anon challengeable law in the physics realm, to see what’s wrong with it and how to solve it, one should understand a little thermodynamics and spiritualism.
Entropy and Carnot engine, low temperature making:
- Any macroscopic system is thermodynamic system.
- Thermodynamic parameters such as pressure, volume, temperature and magnetic field specify thermodynamic states.
- All thermodynamic parameters specify a thermodynamic state.
- Equilibrium is a state when the system ceased its changing with time, when there is no probability. If there is only material, then the heat must be converted constantly from high temperature source to a low temperature source, work would constantly be converted into heat, it is the second law of thermodynamics. The second law of thermodynamics is the conservation of entropy, it is true when there is only material, but actually the process should be probabilistic, i.e., there is possibility of the process that reverses the second law to happen, because spirit does exist and it can create new material which would create plus, minus or zero entropy to the system, and second law isn’t totally wrong, it is just the case when there is only material and everything is deterministic.
- means a state when the system is in equilibrium, reducing one degree of freedom. A point in phase space could represent each equilibrium of a system, and the equation of state makes the points to form a shape in the phase space. In state transformation, when the change of state is so slow that it has enough time to reach equilibrium, it is quasi-static. Under the second law of thermodynamics, reversible transformation is quasi-static since there can’t be the process in the second law happened, so it is always in equilibrium, otherwise it is irreversible. However, some quasi-static process is not reversible, such as the free expansion of gas, it won’t change its temperature when expanding without doing any work, according to Joule’s experiment, however this should also be predictable by relevant theories, it should be proved first then verified by the experiments.
- A process could only be reversible if the transformation retraces its history in time. Reversible transformation is quasi-static so it would be a continuous path.
- , infinitesimal transformation. Heat is what caused the increases of temperature while no work is done, and the change of temperature is different with the same when the manner of heat transferring is different. are for transferring heat with constant and respectively. Thermal isolated is called adiabatic.
- Extensive quantity: it is proportional to the amount of substance in the system. Intensive is contrast to extensive, and thermodynamic quantities are either extensive or intensive.
- Idea gas is the idealization of the universal behavior when all the gases are sufficiently dilute. Each equilibrium has a fixed temperature and when the two equilibriums doesn’t exchange heat simultaneously when they contacted, they are at same temperature. Boyle’s law shows that ideal gas has such equation of state for constant temperature, . Equation of state of an ideal gas
(1)
defines temperature scale. It shows that for constant quantity of the material, the temperature is proportional to and . Measure at points which water boils and freezes, plot them and connect them with a straight line, the abscissa is the scale of temperature, divides the proportionally and the resulting scale is the ideal gas temperature scale, any other material can contact with idea gas to have equilibrium therewith so that they have same temperature, the equilibrium of heat defines temperature here. It could also be proved that ideal gas temperature scale is same to Kelvin scale () later.
(2)
is equivalent to ,.
However, all the statements above are true when and only when there is only material, and actually there is not only material, but also spirit and everything is probabilistically created by the spirit. The existence of spirit so as to its probability could be proved. if there was only material, everything must be deterministic thence there can’t be any material created, i.e., material itself can’t create material; however, material does exist, therefore if can only be spirit other than material which created the material, and it is probabilistic, since the material it created is something never existed before, it can’t be predicted what or whether there is something would be created. Any material was created by spirit and material originally is the reflection of the spirits that created it. One could think that when there is only material, it is indeed true that the heat can only transfer from a high temperature source to a lower temperature one, and only the expansion or compression of the gas can convert heat into work, they are deterministic and therefore the Clausius Statement and Kelvin Statement of the second law of thermodynamics are true respectively. However, material itself is totally probabilistically created, everything is created by spirit, when and only when there is a soul did something purely it likes not determined by any material could something new be created, and it is material because it virtually does exist, existence is the essence of material. Anything is totally from the probabilistic spirit, everything is just an eigen state of the spirit who created it, so anything exists would always have possibility to convert to other things hence everything always could be converted to other things probabilistically, that is what is the saying that bad could be converted to good, bad is just a lack of good. The process of the second law of thermodynamics could be probabilistically reversed by probabilistic effects such as the quantum field fluctuation could evaporate a black hole’s mass which is impossible as no material could escape from the black hole, thermal- or photovoltaic cells1 and photosynthesis can partly convert heat directly into stored energy without any other change, man is producing minus entropy by producing such cells and plants, and the entropy of the man who did that could be reduced by his learning and exercising, by doing what he likes, same to Maxwell’s Demon, the spirit of man is free and determined by himself, it is more than material and purely probabilistic, it can produce purely plus or minus entropy probabilistically, just depends whether he likes. What already existed can’t be eliminated, but could be converted to other things, as mentioned in Marxism and ancient Chinese philosophy. An equilibrium is the stable state when there is only material and no spirit, therefore no new material created and everything is deterministic. The free gas expansion is also irreversible when there is only material; however, factually there is not only material but also spirit, i.e., it is probabilistic.
The thermo dynamical laws can be regarded as mathematical hypothesis to construct a model. Though it ignores the atomic structure of matter so would fail in atomic domain, thermo dynamic laws are factually restrictive, it enables one to draw rather precise and far-reaching conclusion from seemingly commonplace observations. Equation of state is a regular function is factually restrictive.
The first law of thermodynamics is energy conservation, is same to all transformation from a given initial state to a give final state, for each state has certain internal energy ,is exact differential. Choose an arbitrary fixed state as reference () and the internal energy of any state is in any transformation that leads from the reference state to the question state. The saturation property of molecular forces makes an extensive quantity. The first law could be written in form of differential
(3)
, for the heat absorbed and work the system did to the outside depends on the process, they have no certain value at each state of the system therefore use instead of , it is not exact differential.depends only on the limit of the integration regardless of the path, while or doesn’t share such property. The differential of internal energy could also be written as
(4)
(5)
(6)
(7)
(8)
(9)
(10)
where is enthalpy of the system.
Free gas expansion, when there is vacuum, free gas is always tending to fill it. Since the gas has no pressure to overcome, ignoring the energy loss when the gas hit the boundary of the container, the internal energy of the gas didn’t change, therefore the temperature of the gas doesn’t change, it is a quasi-static process since the gas is always in equilibrium, there is no process of the second law of thermodynamics happened, but there is free gas expansion, therefore it is irreversible, it can’t recover to its initial state without any work and heat releasing or other changes, only when there is spirit could the process solely reverse. The free gas expansion doesn’t change its temperature while the internal energy also doesn’t change, this shows could be a function with temperature as its only parameter; therefore,
(11)
,heat capacity could also be defined as
(12)
for constant volume heat capacity, as ,heat capacity with constant pressure is
(13)
(14)
.
Material alone can’t reverse the second law of thermodynamics. when there is only material heat could only transfer from a higher temperature to a lower one, work can only be converted to heat since free gas expansion is irreversible and work can only be done by the expansion of the gas, recovering the gas to its initial state needs compression with non-zero pressure and the internal energy increased by the work must be absorbed by a lower temperature reservoir they are objective laws of the material and deterministic; thereby entropy can’t reduce, entropy essentially is a quantity to measure how much potential there is of the process in direction of the second law of thermodynamics could happen, as proved later. If there was only material, it is deterministic and the reaction can only go along with the direction of the second law of thermodynamics, as the facts in experiments.
When an ideal gas expanded freely and isothermally, its internal energy doesn’t change, because there is no pressure therefore work it would do. Anyway, the work must be saved as heat if the system returned back to its initial state, for there must be pressure to compress it back and that exchanges work with the system, the internal energy it gained from work must be release to recover the system back to its initial state. The work can always make gas expand or shrink with pressure and thereby change its temperature since the work was converted into its internal energy, but heat naturally can only diffuse from high temperature reservoir to a lower temperature reservoir, this makes the work can only be reserved as heat if there is only material when the second law of thermodynamics is valid, otherwise this could be probabilistically reversed if a soul did something probabilistically.
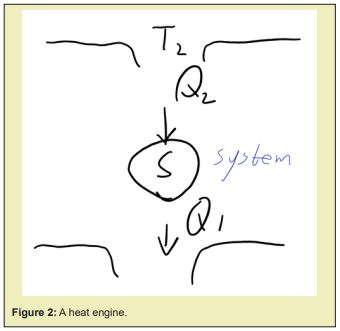
The two statements of the second law of thermodynamics are equivalent, heat could be solely converted to work if the heat was solely converted from a low temperature reservoir to a higher temperature one, and vise versa. This could be proved, if Kelvin Statement was false, the heat could be solely converted to work, then the work solely converted from heat can heat a reservoir with higher temperature compared to previous reservoir where the work came from, such as adiabatically compressing a system and let it contact with another heat reservoir to release proper amount of heat to enable it to come back to its original state naturally when being adiabatic again after the heat exchange, or directly extract the heat used for a inverse Carnot cycle which uses the work from outside to convey heat from a low temperature reservoir to a higher one, thereby the heat was solely converted from a lower temperature reservoir to a higher one, the net result is that the heat was solely transferred from a low temperature reservoir to a higher one, hence Clausius Statement is false; if Clausius Statement was false, the heat could be solely extracted from a colder reservoir to a hotter reservoir, then it can be used to expand a system isothermally to let it output proper amount of work and return the heat it absorbed to a colder reservoir to make it recover to its initial state thereafter when being adiabatically compressed again, this is the same engine as the engine that
- absorbs from ;
- rejects to ,;
- Performs work
, as Figure 2 shows.
It could be proved that, if . When there is only material, only the expansion or compression of gas could exchange work with outside, and the energy could only come from the high temperature reservoir .The internal energy of gas is solely dependent on temperature, its temperature would feel like to recover to its initial temperature as its expanding which output work to outside, the heat it absorbed was converted to work. And since the free gas expansion is irreversible when there is only material, it can’t recover to its initial state if it did works by the heat it absorbed, it must be compressed with pressure which would increase its internal energy, therefore there must always be a part of heat it absorbed can’t be converted to work if the gas recovered back to its initial state, i.e., .Even using a Carnot cycle for outputting work to outside, work must be done to recover the gas back to its initial state and that heat must be absorbed by a reservoir of lower temperature.
In total how much heat would be converted to work or absorbed by the low temperature reservoir depends on the process of heat transferring and work exerting, lower temperature makes less work needed to compress and more to expand same volume of the gas, while the higher temperature makes the inverse effect. Using heat sources of different temperature to expand or compress the gas could make different plus or minus work to outside while keeping the volume of the expansion and compression the same, and recover the system to its initial state after working, As long as the temperature of the initial state of the gas is not absolute zero, work must be exerted to it to compress the gas and to make it recover to its initial volume and the work must be converted into the heat therein since the total internal energy increased and those extra heat also must be absorbed by a heat reservoir of lower temperature to keep the system at its initial temperature, since the average temperature in the process is not zero, there must always be compression needed to recover it to its initial state, the compression is always needed when its volume is larger than its initial state and it would rise the temperature of the gas since the internal energy is increased by the work be done from compression, this amount of heat must be absorbed by a heat reservoir of lower temperature. The temperature of the compression must always be above absolute zero so that the system could be heated when its temperature was lower than its initial temperature, otherwise no heat can be input to the system to recover its temperature after compressing it to its original volume, and the gas must be heated by the work done to it, the work added its internal energy and the internal energy is solely dependent on the temperature, those heat increased by the work must be absorbed by the low temperature reservoir during compressing the system to its original volume, and it won’t have volume anymore if the gas’s temperature is at absolute zero though there is no work need to be done to compress it in this case, the gas can’t be recovered to its initial state by compressing since the temperature can’t recover to its initial one no matter how much it was compressed, and the gas actually doesn’t exist in this case, therefore gas still can’t reverse its free expansion without any work or other change, i.e., heat can’t be solely converted to work itself, when there is only material.
If , then and , as proved under purely material condition. When there is only material, only the expansion of gas can convert the heat it absorbed into work, and the internal energy of gas is solely dependent to its temperature, it must have expanded when it recovered back to its initial temperature to convert all heat it absorbed into work, and the expansion of idea gas without applying work from outside is impossible under pure material condition, the free gas expansion is irreversible and there would always be a part of heat can’t be solely converted to work if the system recovered to its initial state, because some work must be exerted back to the system to let the gas recover to its initial volume and the internal energy input thereby must be extracted to keep the system at its initial temperature when its volume recovered. If , the gas must expand to output work which would lower its internal energy so as to its temperature, as the system would recover to its initial state after doing work, it must absorb proper amount of heat from to compensate the internal energy it lost therefor, thence, ;however, the free gas expansion is irreversible under purely material condition, it also needs compression with pressure to recover its initial volume, with temperatures lower than when it was expanding, which makes the system have less pressure when being compressed at the same volume, so that the work be done to the system for the compression could be less than the work output by expanding, to keep and therefore.
If ,and , still , if this amount of work was converted into heat and transferred to , the net effect is exactly reverse to Clausius statement, the second law of thermodynamics can’t be broken when there is only material. If , the system could refrigerate, in this case, it needs to expand first to extract heat from ,output work and then to be compressed and release heat into , as the has lower temperature than , and the work it needs to compress the system to its initial state is more than that was needed when the gas was expanding since which makes the compression have higher pressure at the same volume as the expansion, the system always needs to be compressed in higher temperature than the compression as a whole, that needs more works to compress than to extract heat by expanding, therefore the total work and the heat would be converted from to with a sacrifice of .
However, when and only when there is something else, i.e., spirit rather than material, can solely convert heat into work or from low temperature reservoir to one of a higher temperature, or even create work (energy) or minus entropy directly, could the heat while . The minus entropy and even the energy could be created from null, heat can be converted to work only by the expansion of gas under the second law. while violatesnot only Kelvin statement, but also the irreversibility of the free gas expansion.
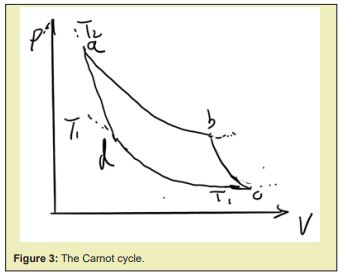
Carnot engine, Figure 3 shows its working cycle, is the reversible engine of using a system to absorb heat from a high temperature reservoir to do work and recover the system to its initial state after a cycle, with two heat reservoirs of different temperatures. The system must release heat to the low temperature reservoir to be compressed to its initial state after outputting work by absorbing heat from the high temperature reservoir , and the low temperature reservoir can only access but can’t be of absolute zero temperature since the system can’t recover its initial temperature after its isothermal compression by contacting to .
A working cycle is separated into for stages, supposing the state of the working system as
- Isothermal expanding with contact to , the system goes from stateto .
- Adiabatic expanding, so that the system could output work with the heat it absorbed from while reach the same temperature to the low temperature reservoir, from state to .
- Isothermal compression with contact to , from to .
- The system should also be able to recover to its initial state by adiabatic expansion from to .
, see the total process doesn’t contain any sole process in or against the direction of the second law, therefore it is reversible.
Carnot engine perfectly obeys the second law of thermo dynamics. Under pure material condition, heat can’t be solely converted to work, or solely transfer from low temperature reservoir to high temperature reservoir, free gas expansion is irreversible, if the working cycle of the engine is reservable, it means that such irreversible process didn’t happen. A Carnot engine with ideal gas as its working material is a reversible engine, all heat the gas absorbed or released from the heat reservoir when the gas was isothermally or adiabatically expanding or being compressed by contacting with the reservoir or the gas stored was all converted as , all expansion of gas is not free expansion since there is always pressure that makes the volume change of the gas to bewhich converts heat into work or work to heat by the changes of the volume, no free gas expansion or the process that heat solely transferred from higher temperature source to a lower temperature one or the work solely converted to heat happened.is always equal to the change of the internal energy so the process is always reversible by reversing the , and the process can go reversely just by reversing at each moment when the gas was expanding or being compressed.
Carnot engine do all things reversibly, the efficiency is
(14)
, and it can be proved that any Carnot engine working at two given temperatures has same efficiency, i.e., the efficiency of Carnot engine is solely determined by the temperatures it was working.
Proof of Carnot’s theorem
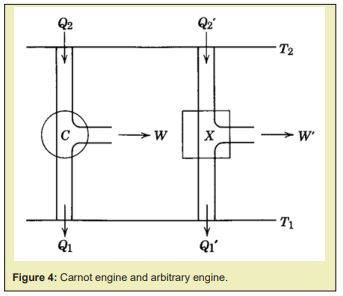
Use two different Carnot engines and which are both reversible but different in heat transferring and the amount of heat that was converted to work, as shown in Figure 4, engine could also be an engine by combining several engines. In a working cycle,
(15)
(16)
, let
(17)
,where and are both finite integers, operate the Carnot engine for cycles and for cycles in reverse, we have
(18)
(19)
(20)
(21)
, under purely material condition when the second law is valid,
(22)
since and as the previous derivation. It could be proved again, under pure material condition, if the system output works and recovered to its initial state, it must absorb heat from higher temperature reservoirs or to compensate such loss of energy, there must be while this is not true, alsosince the expansion of gas (system) is not naturally (purely material) reversible, the system must be compressed if it wanted to recover to its initial state. And it should be compressed in a lower temperature than its expansion so that it needs less pressure (same system, ) when being compressed at the same volume when it was expanding, the work output in the expansions must be larger than the work input in compressions in total, and this makes the system would absorb more heat from than release to in total,as long as the working systems back to their initial states, otherwise all work would be returned back to the high temperature reservoir , no positive work would be output. However, it can be compressed only with lower but not absolute zero temperature, otherwise it can’t recover to its initial temperature when it was compressed back to its initial volume, since the absolute zero degree of temperature needs no work to be compressed while it still needs to be heated by the work done to it to recover its initial temperature when being compressed to its original volume, and the system always needs to release heat taken by the compression into the heat reservoir of lower temperatures to keep its internal energy invariant to its initial so that it could recover to its initial temperature when its volume was recovered; thereforewould make , and .Since, there must be
(23)
; however, both the two engines are reversible Carnot engine, and under pure material condition the second law is valid and it makes, therefore
(24)
, or to make the total process go inversely, the work output would be and it should satisfy
(25)
since , ,as well; thence,
(26)
(27)
this means that any Carnot engine works between two given temperatures have fixed efficiency, and use this relation between the efficiency and working temperatures of a Carnot engine could define a thermo dynamical scale for temperature, the efficiency is a quantity that could be solely determined by the two given temperatures between which the engine is working, it is a function of the two given temperatures. Supposing a Carnot engine , its efficiency
(28)
is solely determined by the two temperatures between which the engine is working, where andare the heat absorbed and released at its and temperature reservoirs respectively, and , since the efficiencyis a function of the two given temperatures,is also a function thereof
(29)
, also, supposing another Carnot engine works between where andare the heat absorbed and released at its and temperature reservoirs respectively, , Figure 4 could be used again here, its efficiency is
(30)
; thereafter,
(31)
, conjugating the two Carnot engines to work in one line, letting the absorbed at after a working cycle of totally released from into ’s working cycle, thereafter when the two cycles were finished, the total effect is a new Carnot engine (reversible engine so it is Carnot engine as well, any reversible heat engine was called as Carnot engine and Carnot’s theorem still work on any Carnot engine) working at , and it has efficiency as
(32)
, and is a function of
(33)
, as
(34)
, that is
(35)
, therefore, is solely determined by as the engine by conjugating and is still a Carnot engine working at and its efficiency is solely determined by the two temperatures where it is working at; thereafter,
(36)
, it is not determined by , and this means
(37)
, since any Carnot engine working between same temperatures have same efficiency, it is true for any Carnot engine that
(38)
,and could be used as a scale of temperature, called thermo dynamical temperature scale.
Since the system can’t recover to its initial state without any work or other changes, i.e., the efficiency of Carnot engine can only access but can’t reach 100%. When the Clausius statement was false, the heat that wasn’t converted to work would be solely transferred to the high and the net effect would be that the heat was solely converted to work and violate the Kelvin statement.
In addition, if using ideal gas as the material for working in the system of Carnot engine , in isothermal expanding, where is a constant, the work be done is the heat it absorbed since the internal energy is solely determined by the temperature, the internal temperature doesn’t change so all heat absorbed was converted to work. Supposing the state of the system working in is ,,,are the pressure, volume and temperature of the system respectively.
Firstly, the system would isothermally expand by contacting where it absorbs heat from, expanding from state to and the heat absorbed by the system there from would be
(39)
, then it would adiabatically expand to and output more work; thereafter, it would be compressed isothermally by contacting where it releases heat into, and it would be isothermally compressed from state to state by contacting where it releases heat into, the heat released thereto by the system would be
(40)
. State should be able to recover to its initial state by adiabatic compression thereafter which would make it to recover to its initial state.
For ideal gas, according to the first law,
(41)
, and in adiabatic process ,, and the internal energy is solely dependent on temperature , where is also solely determined by temperature
(42)
, define , as before, , and differentiate the equation of state of ideal gas ,
(43)
,
(44)
(45)
(46)
(47)
, that is, the adiabatic process of ideal gas is
(48)
, where is a function solely determined by temperature ,
(49)
(50)
(51)
, using the equation of state of ideal gas
(52)
, for adiabatic process of ideal gas. As the process from toand from to are both adiabatic, there are
(53)
(54)
, i.e.,
(55)
; therefore,
(56)
, this defines another temperature scale by ideal gas, called ideal gas temperature scale; comparing it with thermo dynamical temperature scale,
(57)
, it proves that the thermo dynamical temperature scale is same to ideal gas temperature scale,
(58)
. And writing and both in form of the heat that absorbed by the system from the reservoirs (releasing means absorbing minus amount of heat it released), there is
(59)
for any Carnot engine. And for any reversible cycle , supposing the system absorbs heat from each (a heat reservoir of temperature ) to do works and recovers to its initial state after a cycle, to calculate
(60)
, supposing there is an auxiliary heat reservoir where is its temperature, and there is also an auxiliary Carnot cycle between and before the system went from process to its next process, it absorbs again from and then absorbs from to finish the cycle, since it runs an Carnot cycle, there is
(61)
, summing all up
(62)
, i.e.,
(63)
, are all heat released from . Since the system recovered back to its initial state, and all heat absorbed by the reservoir was absorbed away by from all reservoirs, and both the processes of Carnot engine that absorbs heat from each reservoir as well as the reversible cycle are reversible, the total process is reversible, therefore , since no work was solely converted to heat otherwise the process can’t reverse. Anyway, is also impossible in reversible process, since the heat was solely converted to work, the process can’t be reversed. Actually, if any process that solely converted work to heat or the heat solely transferred from a high temperature to a lower temperature happened in the total process, there must be , the process is reverse to the second law of thermodynamics, and must make the processes irreversible. In conclusion, since the processes are reversible and natural, there must be
(64)
; therefore,
(65)
for any reversible process, i.e.,
(66)
and this could define a quantity: entropy,
(67)
, if any process that is in the direction of the second law of thermodynamics happened, then , as ,or reverse to the direction of second law of thermodynamics, then ,, scales how much potential could the process of the second law of thermodynamics happen. Under pure material condition, since , minus entropy is the potential that the process in direction of the second law of thermodynamics could happen.
However, all what was analyzed above are under the condition that there is only material, but spirit does exist and it is what created the material. Spirit can do things probabilistically, what it did probabilistically virtually does exist since that is not determined by material, it is something never existed before, therefore is exactly what material is. It was because in general no spirit who can do things purely it likes not determined by material that makes no transformation has sole effect that could reverse the second law of thermodynamics; however, what makes a Carnot engine itself must be probabilistic, i.e., must be spiritual, otherwise no minus entropy would be created if there is only Carnot engine, i.e., if there was only material, Carnot engine itself is something deterministic and can’t create minus entropy, while all entropy it uses does exist, there must be something created those minus entropy. There is just neither creation nor consumption of minus entropy, a Carnot cycle could go reversely if the work it did wasn’t converted to other forms of energy. The heat can only go from a hotter reservoir to a colder one if there is only material, the heat can’t go back to its initial hotter reservoir if it was from works, work could be converted to heat by compressing a gas system first and let it release the internal energy it got from the work to recover its initial state by a adiabatic expansion thereafter, but the heat can’t recover to be work again since the heat transferred from a high temperature source to a low temperature source, this process can’t be reversed, the second law of thermodynamics is valid when there is only material. Actually, there is not only material, but also spirit, and it allows process that is reversed to the second law of thermodynamics to happen probabilistically, when and only when spirit did things it likes which is not determined by material, i.e., probabilistically, it would create something new that virtually does exist, i.e., it would create new material, that must reverse the second law of thermodynamics which would happen when there is only material. The creation of new material, i.e., whether a soul would do what it likes is probabilistic, and it is of the inverse direction of purely material process, that is, totally inverse to the materialism, there for the process that inverse the second law would happen probabilistically determined by whether the soul would do something purely it likes probabilistic but not determined by material which rebels what it likes.
Carnot cycle is just a reversible transformation cycle. Actually, anything deterministic, i.e., only material can't create any minus entropy but minus entropy does exist, there for there must be spirit which is probabilistic (indeterministic) that created minus entropy, i.e., it is spirit doing something purely it likes not determined by material that created new material with minus entropy. Carnot engine is just a reversible cycle for heat-work conversion, all processes are purely material, no minus entropy created or annihilated in a cycle, therefore the entropy is conserved if no process in direction of the second happened, i.e., if neither work was solely converted to heat nor the heat was solely transferred from high temperature reservoir to low temperature reservoir.
Photosynthesis captures the energy from sunlight to synthesize carbohydrates which is the energy storage for cells,
(68)
it is the main route of energy producing for the earth, the whole biosphere on the earth needs its energy supply. It assembles carbon dioxide and water into carbohydrates. The respiration is the reverse process to photosynthesis, it releases the energy stored in carbohydrates mostly into which is the energy current for the cell, available to drive many biochemical reactions for the metabolism of the cell.
Photosynthesis use visible light as its energy source and the visible light is enough to excite the electrons in the molecules there for, this is like a kind of photoelectric effect. The photosynthesis mainly consists of two stages, light cycle and Calvin cycle.
The light reaction occurs in chloroplast thylakoids, the photon in available frequency excites the electrons in chlorophylland accessory pigments and the excited electrons could
- split the water molecules to be oxygen , mostly released into the atmosphere;
- synthesize using its energy carried;
- combine electron acceptor with hydrogen from splitting water to form ;
, light reaction produce oxygen more than the plants need and whichcarry energy from those excited electrons.
The light cycle is shown as Figure 5, the antenna complex in the photo system Ⅱ absorbs energy from light and deliver it until it was passed to one of the special chlorophyll molecules called 3 in the reaction center of photo system Ⅱ, then excites the electrons in the reaction center to the first electron transport chain, a significant part of energy in these electrons would be captured in molecules of , though a part of them would dissipate as heat which increases the entropy. Thereafter the electrons in the chain would transfer to the reaction center of the photo system Ⅰ and replace the electrons transferred there from to the second electron transport chains, through which the electrons would reach reductase where they are combined with protons and to form . The electron transport chains also branch part of the energy therein to pump protons from the stroma into the thylakoid lumen, where also lie the enzymes for the photolysis which split water into electrons, protons and oxygen, it is where the photolysis happens. The electrons and protons from the photolysis and the proton pumping replace the electrons lost in the photo system and provideand needed to synthesize . The photolysis is a entropy reducing process, since the photons were fixed and stored into the and as chemical energy, since they provide potential for the processes in the direction of the second law of thermodynamics.and are energy carries available for the further carbon-fixation reactions which is called Calvin cycle.
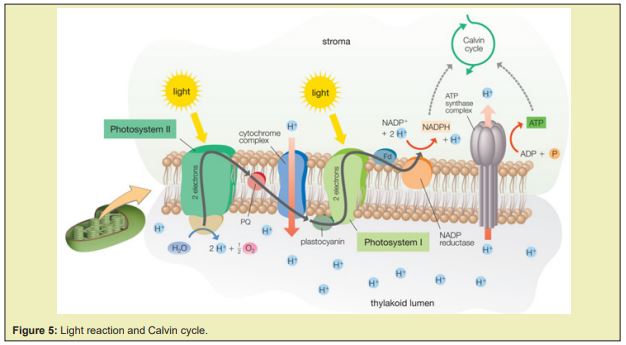
The Calvin cycle is the series reactions that reduce the carbon dioxide from the atmosphere into sugars and other carbohydrates, it is the only known pathway there for, see Figure 6.
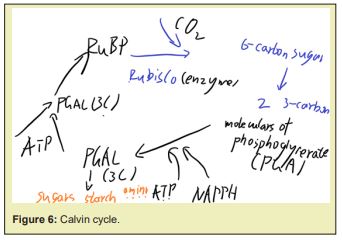
It uses an enzyme (enzyme ribulose 1,5-bisphosphate carboxylase/oxygenase) to combine the carbon dioxide with a 5-carbon sugar (ribulose 1,5-bisphosphate) and form (3-carbon molecules of 3-phosphoglycerate), which could be reduced to with the energyand the reducing power of the and gained from the previous light reaction. The would be partly rearranged to regenerate to compensate the amount of which were just usedto synthesize them, but there must be remainedemerged, since the absorbed were also used to produce the , and those emerged from the cycle could be used to make sugar or starch, those retained in the chloroplast would be converted to starch, or made into sucrose if exported from the chloroplast. And these carbon-fixations transport the entropy from and intothe sugar, starch or other carbohydrates synthesized therefrom, the potential of the process in direction of the second law of thermodynamics to happen was transported therewith.
In summary, through the light reaction and Calvin cycle in photosynthesis, the entropy, the potential of the heat solely being converted from high temperature to low temperature or from work to heat, of the photons from the sun was saved in the molecules of and first and then stored in the carbohydrates synthesized using the Calvin cycle, such as sugar and starch. And this potential of entropy almost feed the lives of the whole biosphere on the earth, the life our human need, as life lives on minus-entropy, at least only with such potential could we convert the energy we gained in useful form, the movements of lives actually are same to Carnot engine, and life is movement. It is worth mentioning that Boltzmann has more fundamental and essential understanding about entropy, by his formula , theoretically proved it.4
The photosynthesis in plants absorbs sunlight, and and converts the energy in the sunlight into the chemical bonds etc. in the carbohydrates produced thereby, the entropy from the sun was also stored therewith so that it could be used when one needs, i.e., to do useful works. Since the entropy, i.e., the potential of the heat solely transferring from high temperature to low temperature or being converted into works, was stored in the carbohydrates synthesized there from, a significant part of energy from the sun was saved into the chemical bonds in these molecules instead of dissipating into heat and therefore temperature lowered down. This is what caused heat island effect, the photosynthesis in the plants generates minus entropy when the photons passing by and fixes the energy in the light from the sun into chemical energy which was saved in the carbohydrate synthesized through the light reaction and Calvin cycle and thereby the heat was stored in products of the photosynthesis instead of being released into the environment.5-11
In Metropolises today, heat island problem deteriorates people’s living environment and makes the space where people live extremely hot, sometimes unacceptable and the city was full of air conditions to keep the indoor space partially habitable. People also thought up many measurements to solve the problem such as dyeing the road to be white, planting the skyscrapers with trees etc.; however, the white road would also reflect sunlight into driver’s eye which makes him can’t see anything, and it is expensive to fix trees on skyscrapers safely with enough sunshine. And some Metropolises are also hard to afforest to lower the temperature of the city, such as Xi’ān in Shǎnxī Province in China, makes it also hard to resolve haze problem by afforesting,2 the temperature in urban region is really hard to cool down by barely modifying its internal design, the city itself is a heat generator compared with the afforested region around it. What’s worse is that the excess use of air condition also is a significant cause that raises the city temperature, for they are reversed Carnot cycle that converts heat from low temperature to high temperature with a sacrifice of the work exerted from the outside solely dissipating into the heat reservoir.
However, as the city itself is a heat generator because of the lack of afforestation, and this is what makes a city, it actually could be positively used as a pollution outlet where people didn’t live, and intensively use it as a center for industry, leaving hospitable (safe and healthy) spaces inside for people’s working while quarantine from the outside industrial region where the pollution and heat concentrated, and build up some safe high speed tunnels for workers to enter and exit their working places in the industrial city; thereby, utilizing the heat island effect to rearrange the city design, interchange the arrangement of industry and residency in the city as center for industry and working while the suburban for living and education with as much more afforestation as possible therein; there after, the air stream around the city would be attracted towards the center of the city by the heat island, not only the region where people live became healthier and cooler, more habitable, but also the heat island would take the pollution including haze together to be expelled out of the city. In this way, the heat island is alike a huge chimney for the city to expel the industrial pollution including haze out of the city in high rate while didn’t affect people, the people would live in a region separated from the industrial pollution protected by the afforestation layer between the suburban and industrial city center.
Positively using heat island effect to redesign the city, arrange the center of the city for industry separated from the residential region which was all removed to the suburban region, and afforest the suburban regions as possible, the places where people are living would become cooler due to the photosynthesis by the plants, through which the sunlight would be absorbed and converted into foods instead of dissipated heat as what happened in city, the photosynthesis directly generates minus entropy which converts the heat in the sunlight into foods; thereby, the temperature around was lowered, the pollution of the industry can be extracted and insulated from the residential region by the heat flow to the city center and afforesting can be applied again to eliminate haze in people’s residential region and modify the environment where people live to be more healthful with scientific plantation. In addition, people should not stay in their working places in the city where is the industrial center for too long, and their spaces in the city should be quarantined from the outer industrial area but connected to the suburban region where the afforestation is abundant to keep the inside safe healthy, and they can enter or exit their working spaces fast there.
Thanks to the editor for her patience waiting, thanks to the journal team. Thanks to the professors who taught me many useful things, the probability inspired me to prove the spiritualism successfully, and it is necessary for further understanding in science so as to its progresses, material is totally generated by spirit, the objective laws of material could be subjectively understood by man, one can control the nature there by. I love physics, it is something can absolutely never be castrated from my soul. Thanks to the society and my parents, they gave me chance to study and thrive.
Author declares that there is no conflict of interest.
None.
- 1. ZHANG Jiayi. Modification of X-ray Generator by Energy Reusing. Instrumentation. 2020;7(1).
- 2. Jiayi Zhang. Afforesting as a Way to Eliminate Haze and to Fertilize Plants. Glob Scient Res Env Sci. 2021;1(2):1–5.
- 3. Graham LE, Graham JM, Wilcox LW. Pearson New International Edition. Plant Biology. 2014;106–119.
- 4. William H. Cropper. Great Physicists. Oxford University Press. 2004;179–181.
- 5. Kerson Huang. Statistical Mechanics. John Wiley & Sons. 1–13.
- 6. Zhicheng Wang. Thermodynamics and statistical physics. High Education Press. 33–38.
- 7. The Urban Heat Island Effect - YouTube
- 8. Photosynthesis - YouTube
- 9. Urban Heat Island Effect: What It Is and What We Can Do to Fix It - YouTube
- 10. What is the heat island effect? - YouTube
- 11. 3 Cool Ways to Cool Our Cities - YouTube

